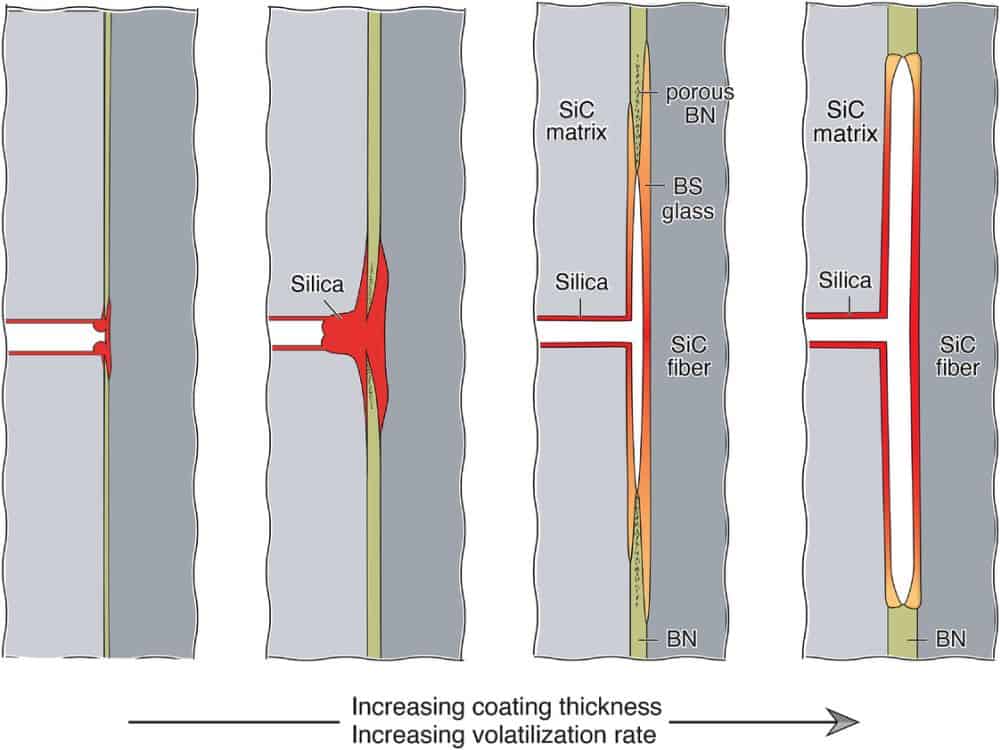
[Image above] Schematics illustrating transitions in recession and closure behavior as the coating thickness and volatilization rate increase (from left to right) in SiC-BN-SiC ceramic matrix composites. Credit: Christensen and Zok, Journal of the American Ceramic Society
If you’ve flown during the past few years, chances are you took a plane containing ceramic matrix composites (CMCs).
CMCs are a special type of composite material consisting of ceramic fibers or whiskers embedded in a ceramic matrix. Compared to nickel-based superalloys, the current main material used in the hot zones of an aircraft, CMCs have a much lower density (about one-third of the superalloys) and higher temperature capabilities (up to 1,400°C).
Silicon carbide-based CMCs first took flight in 2016 as replacements for nickel-based superalloy turbine shrouds in the new LEAP engine developed by GE Aviation (now GE Aerospace) and Safran Aircraft Engines. Since then, CMCs have found their way into other aircraft components, for example, as combustor inner and outer liners as well as nozzles in the GE9X engine.
Though CMCs have entered commercial operation, there still are some knowledge gaps regarding certain aspects of these materials that scientists look to answer. Specifically, the internal oxidation processes of CMCs.
Most hot nonoxide compounds are extremely susceptible to surface oxidation. That is why they are coated in environmental barrier coatings to protect against oxidation.
However, if the environmental barrier coating delaminates or cracks, the CMC may be exposed to hot combustion gases, resulting in oxidation. As such, it is important for manufacturers to understand the oxidation processes so they can properly predict the lifetime of CMCs.
While there is a standard model for analyzing the internal oxidation processes (see sidebar below), critically assessing the event sequence and mechanistic elements of the standard model by direct observation is challenging.
After oxidation, open gaps will appear between the composite constituents. These gaps make the material highly friable and difficult to prepare for analysis with transmission electron microscopy or scanning electron microscopy, both common analysis tools used by ceramic scientists.
In a recent study, two researchers from the University of California, Santa Barbara, attempted to prepare samples for scanning electron microscopy analysis using a method based on ion-mill sectioning/polishing.
The researchers are graduate student researcher Victoria Christensen and Frank Zok, ACerS Fellow and Distinguished Professor of Materials. For their study, they used unidirectional minicomposites consisting of 800 Tyranno ZMI silicon carbide fibers coated with boron nitride and silicon carbide via chemical vapor deposition.
They oxidized the minicomposites by placing 1-centimenter-long specimens in a quartz tube furnace at 1,000°C for 12 hours in flowing, dry air (water content ≈ 10 ppm). Scanning electron microscopy analysis was performed on the samples both before and after oxidation. Additionally, phase distributions were obtained from secondary electron imaging and backscatter electron imaging modes, as well as energy-dispersive spectroscopy maps.
The standard model for internal CMC oxidation processes
The oxidation process of silicon carbide composites (SiC/SiC) with boron nitride (BN) fiber coatings occurs through multiple coupled steps involving mass transport, reaction, and volatilization.
– Internal oxidation. When oxidants gain internal access to the CMC, typically through matrix cracks, both BN and SiC will oxidize and convert into boria and amorphous silica, respectively. At moderately high temperatures (500–1,000°C), these oxidation products will mix to form a low-melting point, low-viscosity borosilicate glass.
– Viscous flow. Oxidation of BN and SiC is accompanied by a volume expansion: about 60% in the conversion of BN to boria and about 120% in the conversion of SiC to amorphous silica. The volume expansion combined with the low viscosity and confined space leads to viscous flow of the evolving oxides into adjacent available spaces.
– Volatilization. At the same time as viscous flow, boria volatilizes either directly from the free surface as a boron oxide vapor or after reaction with water as a borohydroxide vapor.
– Composition evolution. Volatilization depletes boria at the reaction site, and boria subsequently diffuses from distal regions toward the free surfaces. If the rate of volatilization exceeds the rate at which boria is replenished, the oxide becomes silica-rich; its viscosity then increases, slowing further flow.
– Recession cessation. After enough time, the silica scales on the fibers and the matrix impinge on one another. If impingement occurs uniformly around the fiber, further transport of oxidants to the reaction zone is slowed and may eventually cease. Although this outcome is desirable, it comes at the expense of creating strong bonds between the fibers and the matrix. These bonds lead to high stress elevations in the fibers during subsequent unloading/reloading or thermal cycling, which compromises composite strength and toughness.
Notably, the oxidized test specimens consisted of both broken and unbroken samples so as to provide a clearer picture of the internal oxidation mechanisms. The unbroken sample featured a polished transverse cross section and was sectioned longitudinally for examination. The other sample was broken in a tensile test and was sectioned longitudinally for examination as well.
According to Christensen and Zok, this experimental design yielded “observations of the oxidation processes with arguably unprecedented fidelity.” Specifically, they gained new insights into the event sequence leading to both coating recession and gap closure.
In general, they determined that when the coatings are thin and volatilization is slow, only a small amount of boron nitride needs to be consumed before the gaps for gas transport are sealed and the process effectively ceases. For somewhat thicker coatings, the amounts of boron nitride removed and oxide produced are greater, and the time required to seal the recession gap is longer.
On a more detailed level, their observations revealed the closure process involved the transport of oxidants along the coating/fiber and coating/matrix interfaces ahead of the recession front. “Gap closure therefore occurs in subsurface regions near the recession front rather than at the matrix surface,” they write.
Additionally, even though the water content in the dry air was at mere ppm levels, volatilization by reaction of boria with water to form HBO2 gas was sufficient to cause measurable amounts of recession even following relatively short exposures at 1,000°C. If water was present in even larger concentrations, it would also accelerate silica formation “as water is a more potent oxidant of SiC than oxygen,” Christensen and Zok write.
Based on these observations, they determined that the current standard model for internal CMC oxidation processes is only applicable to the extreme case where coatings are very thick and volatilization is rapid. In every other case, the standard model overpredicts recession lengths by a significant margin due to three underlying assumptions.
- Gap closure occurs at the top of the recession channel, not at its base.
- The SiC oxidation rate is unaffected by the presence of boria.
- The oxidation-induced volume expansion is accommodated purely by displacement normal to the oxidizing surfaces, without physical constraint and without flow parallel to the surfaces.
“The current experimental observations call into question these assumptions and point to the need for more refined models to capture the observed behavior,” they conclude.
The paper, published in Journal of the American Ceramic Society, is “Insights into internal oxidation of SiC/BN/SiC composites” (DOI: 10.1111/jace.18834).
Author
Lisa McDonald
CTT Categories
- Aeronautics & Space
- Basic Science