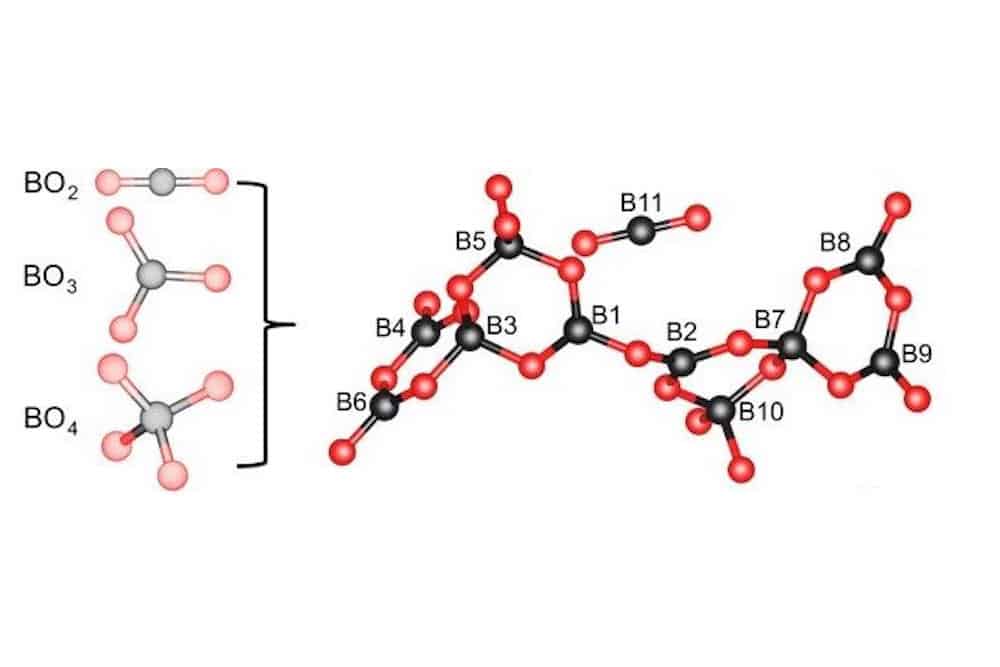
[Image above] Crystal-structural features of K5Ba2(B10O17)2(BO2). This compound is the first to contain all three borate structural units simultaneously, namely linear BO2, trigonal BO3, and tetrahedral BO4. Credit: Huang et al., Nature Communications (CC BY 4.0)
From adhesives and lubricants to agriculture and energy, borates have a role in a wide variety of applications.
One area with a large role for borates is nonlinear optics. As we explained in a previous CTT, nonlinear optical materials can convert light to other frequencies, thus allowing creation of low-power and compact light sources that operate in wavelengths typically requiring more power and larger devices.
Alkali and/or alkaline earth metal borates (A-borates) are frequently used in nonlinear optics because of their high transmittance at nanometer wavelengths combined with higher damage threshold. However, as researchers push to create ever smaller optical devices, they are running into a problem with the current A-borates.
“…compounds with a higher absorption edge energy (lower wavelength), larger birefringence, and larger nonlinear optical coefficient are required to enable smaller optical devices leading to new uses and increased efficiency in industrial and scientific applications,” researchers write in a recent open-access paper. However, almost all of the current A-borates contain borate in the traditional triangular BO3 and tetrahedral BO4 unit structures—structures that do not allow the A-borates to achieve such enhanced functionality.
The researchers comprise an international team led by professors Shilie Pan (Xinjiang Technical Institute of Physics & Chemistry, Chinese Academy of Science) and Kenneth R. Poeppelmeier (Northwestern University). In the paper, they argue that exploring borates arranged in other structural configurations will allow design of nonlinear optical materials with enhanced functionality.
The researchers chose to explore A-borates containing linear BO2 structural units. This structural unit is rare and has only been observed in apatite-type A10(PO4)6–x–y(SiO4)x(BO4)y(BO2) and Gd4(BO2)O5F to date. “Thus, among the thousands of borates, there is an absence of studies of borate-based optical materials with BO2 units,” they write.
Using a high-temperature solution method in an open system, they fabricated single crystals of K5Ba2(B10O17)2(BO2) containing BO2, BO3, and BO4 units. They characterized the structure by using density functional theory-based nuclear magnetic resonance spectroscopy; infrared spectrum measurements and vibrational modes calculations further confirmed the borate structure.
They calculated that K5Ba2(B10O17)2(BO2) has a birefringence of 0.062@1064 nm, which is near the maximum threshold (~0.07@1064 nm) of A-borates comprising only BO3 and BO4 units. Despite the relatively high value, the researchers felt the birefringence enhancement was small for a material containing BO2 units “due to the low density (1/21) of the boron sites.”
They postulated that a borate with a high density of BO2 units would have a larger birefringence, and they tested this hypothesis by investigating a theoretical structural model of K(BO2), a structure comprising solely BO2 units. They calculated that K(BO2) would theoretically have a birefringence of 0.18@1064 nm—a value larger than any previously reported A-borate, including Ca(BO2)2, which contains borate in only the BO3 unit structure and has a birefringence of 0.124@1064 nm.
“The predicted birefringence in K(BO2) would lead to higher-efficiency beam splitting in light polarization processes across telecommunications and scientific instrumentation applications and provide enhanced ability to create and control polarized light,” the researchers write. In addition, “The BO2 functionality could enable optical capabilities, specifically in the rarely accessible deep UV range, and thus sets a target for synthetic crystal growth.”
In the conclusion, the authors write that they hope this study will “motivate and provide a methodology for its [BO2 unit] broader study.”
The open-access paper, published in Nature Communications, is “Expanding the chemistry of borates with functional [BO2]− anions” (DOI: 10.1038/s41467-021-22835-4).
Author
Lisa McDonald
CTT Categories
- Basic Science
- Optics


