[Image above] Credit: Joe Doe; Flickr CC BY-NC-ND 2.0
I’ve said it before: Bone repair is a challenge.
Just think about the material requirements of bone. I imagine a bone-seeking-compatible-material dating profile to read like something like this:
Intricate porous living structure seeking biologically compatible material that spurs my own personal growth. I’m looking for the perfect-fit material, one that’s lightweight, porous, stiff, strong, dynamic, and willing to integrate—completely. Must be utterly selfless and willing to vanish when no longer needed. Oh and I like them strong—companions must be able to match my own strength with an ability to support 19,000 lbs.
Not an easy match to make.
So it’s a challenge to repair bone, and even more challenging when that repair needs to span a large defect in a load-bearing bone.
The best approach to date is to cut out a chunk of bone from another part of the patient’s skeleton and graft it into the area of need, a procedure called an autologous bone graft—not the most desirable option for the patient, and not a procedure without fail, either.
There also are existent synthetic approaches for repair bone that use ceramic or polymer scaffolds loaded with growth factors and bone-forming cells to help colonize new bone growth. But these strategies have low mechanical stability and are rather expensive because of the addition of those biological factors.
So new and improved materials are still needed—and that’s where glass comes in. It is anything but ordinary, but can glass really repair bone?
Bioactive glass, invented by Larry Hench, has shown success in repairing bone and beyond. But it can’t fix everything when it comes to broken, defective, or damaged bone. At least not yet.
Now an international group of researchers report that bioglass is going where few materials can—into successful repair of large defects in bone.
The team—including researchers from Shanghai Jiaotong University and Tongji University in China and Lawrence Berkeley National Lab in California—has developed a technique for producing bioglass scaffolds that alone can successfully repair large defects in load-bearing bones.
“This is the first demonstration of a synthetic material that can bridge a large segmental bone defect, without using growth factors or bone marrow stromal cells, in a relatively large animal (rabbit) model,” Qiang Fu, senior author on the paper, says in an email. Fu helped develop the glass scaffolds at Berkley Lab and is now with Corning Inc.
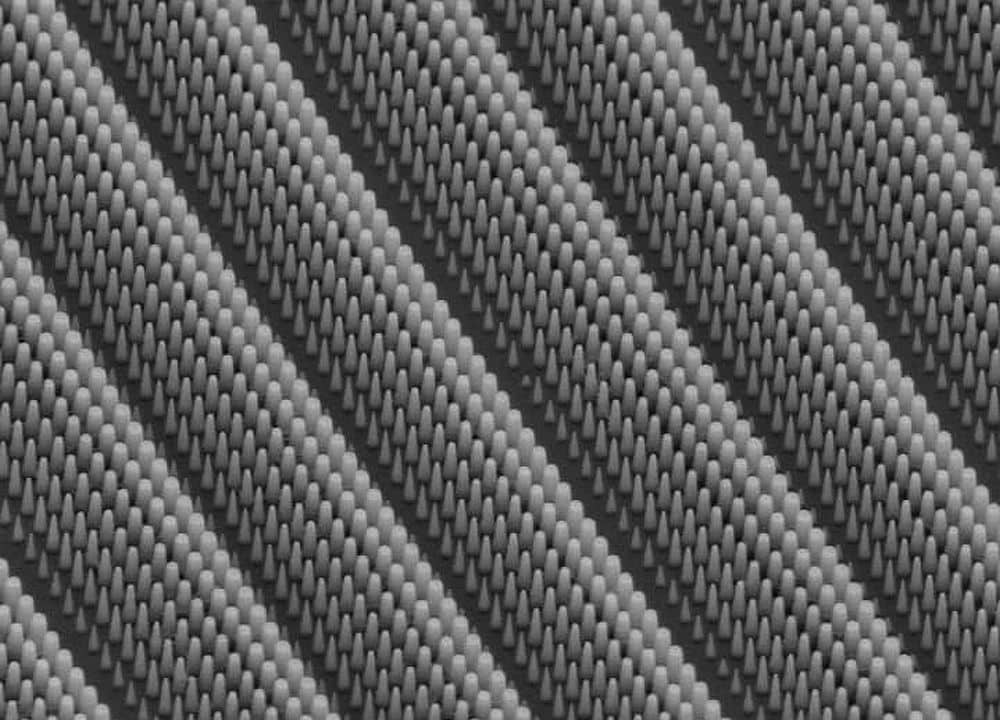
Using direct ink writing, the team fabricated two types of scaffolds with different bioglass compositions, silicate glass 13-93 and borosilicate glass 2B6Sr. The technique, which builds structures layer by layer, allows precise control of microstructure by creating regular pores in the bioglass scaffold.
That porosity is key to the material’s performance when implanted in living bone—holes and tunnels in the scaffold are necessary for cells to be able to infiltrate through the scaffold to lay new bone that will permanently bridge the defect.
Akin to scaffolding on a building, the bone scaffolds offer temporary support during construction—they are installed where work is needed, then removed once the job is complete.
Before even testing how well the scaffolds could integrate with living bone, however, the researchers demonstrated that the strength of 3-mm cubic samples of their bioglass scaffolds were far superior to previously reported scaffolds.
According to Berkeley Lab, the bioglass scaffolds are ~100 times stronger than polymer scaffolds and 4–5 times stronger than ceramic and glass scaffolds with similar porosities. See a graphical representation here.
Such high strength means that the researchers could implant 6 mm x 10 mm samples of the bioglass scaffolds into the middle of rabbit femur bones.
Nine months after the rabbits received their bioglass fix, the scientists took a closer look at how well the bones repaired themselves. Compared to the gold standard autologous bone grafts (which the researchers also performed as a control), the bioglass scaffolds repaired bone equally as well. The researchers could discern no visible interface between the rabbit bone and implant, showing just how well the bioglass supported full bone repair.

Direct ink writing can deposit bioglass scaffolds layer by layer (left), fabricating intricate and porous structures (middle) that can be implanted in bone. (right) Micro-CT image of a 2B63 scaffold implanted in a rabbit femur bone (* = glass scaffold, # = mineralized callus, ‡ = trabecular bone). Credit: Qiang Fu
Rabbit cells had infiltrated the scaffolds, which dissolved to help rebuild new bone with their constituent elements. In the paper, the authors explain, “The release of soluble Si, Ca, P, and Na ions as a result of glass surface reaction and degradation is reported to give rise to the osteoinductive and osteogenic properties in bioactive glass.” The scaffolds resorbed as they did so, leaving behind only repaired bone.”
Perhaps most importantly, the researchers also showed that after nine months, bioglass-regenerated bone was just as hard as normal bone.
“Bioactive glass scaffolds work just as efficiently as the gold standard autologous bone graft in regenerating new bones and promoting the formation of blood vessels,” Fu says in the email.
Although both bioglass compositions performed well, the authors note that 2B6Sr degraded faster and induced more bone formation than 13-93, “an indication that borosilicate glass was more resorbable than silicate glass,” they write in the paper.
Fu says, “The unique combination of high strength, high porosity, and excellent bioactivity is believed to contribute to their superior performances. Our work opens a new avenue for the reconstruction of large bone defects in both large animal models and clinical practice.”
But how do rabbit bones compare to human bones?
Fu says that rabbit bones have more trabecular mass (the inner spongey, less dense part) than human bones, “making them proper models for evaluating the healing of segmental bone defects.”
“Successful regeneration of segmental bone defects using bioactive glass scaffolds in a rabbit model paves the way to the further assessment of their performances in large-animal models like dog, goat, sheep and pig, which have similar bone composition and biology to those of humans,” he adds.
The team is now working on a few avenues in that direction, including testing larger defect sizes in larger animals, preparing for pre-clinical trials, and designing new bioglass compositions that are even tougher and stronger—part of which is funded by Corning—Fu says. “I hope by end of this year we will have some preliminary results from our larger animal model and pre-clinical trials.”
We’ll keep you updated, but it’s looking like these bioglass scaffolds may just be bone’s perfect match.
The paper, published in Advanced Healthcare Materials, is “Bioactive glass for large bone repair” (DOI: 10.1002/adhm.201500447).
Author
April Gocha
CTT Categories
- Biomaterials & Medical
- Glass
- Material Innovations
Related Posts
Sports-quality ice: From pond side to precision Olympic engineering
February 12, 2026