
[Image above] Linalool, one of the main chemical compounds in lavender oil, could help enable the creation of durable and long-lasting sodium–sulfur batteries. Credit: Kotkoa, Shutterstock
After a long day at the office or a hard workout at the gym, many people find there is nothing better to soothe the mind and body than a long soak in a warm bath. To elevate the experience, essential oils in the form of sprays, bath salts, and shower fizzers are frequently added because, despite mixed clinical results surrounding their effectiveness, aromatherapy has grown into a formidable market force alongside the global health and wellness industry post-pandemic.
Lavender is one of the main flowers used for essential oils both currently and historically. It has a long-standing reputation for addressing nerve-related issues, such as migraines and stress, but it also has antimicrobial compounds that can promote faster healing of burns, cuts, wounds, and scrapes.
While these benefits may indirectly contribute to a longer and healthier lifespan, researchers from the Max Planck Institute of Colloids and Interfaces recently found that lavender can help actively extend the service life of sodium–sulfur batteries.
Sodium–sulfur batteries are emerging alternatives to lithium-ion batteries as stationary storage units. These molten-salt batteries use liquid sodium and liquid sulfur for the electrodes, which are more readily available and affordable materials than lithium and cobalt. They also can potentially achieve very high energy densities exceeding 280 Wh/kg, in contrast to the 100–250 Wh/kg energy density of current lithium-ion batteries.
Achieving these theorized high energy densities is hindered, however, by the shuttle effect. The shuttle effect refers to the migration of sodium polysulfides between the cathode and anode during charging and discharging. These polysulfides are formed as intermediates during the sulfur reduction/oxidation process, and their diffusion between the electrodes causes a continuous cycle of active material loss, thus leading to capacity fade and reduced battery performance.
To mitigate the shuttle effect, nanoconfinement has emerged as a promising solution. Nanoconfinement involves using nanoporous materials or structures to confine a battery’s electrolyte, which helps control the formation and discharge of unwanted intermediates, such as sodium polysulfides.
As described in a recent open-access paper, the Max Planck researchers performed inverse vulcanization and thermal condensation on a mixture of elemental sulfur and linalool, one of the main chemical compounds in lavender oil, to obtain a hybrid sulfur–carbon nanostructure with pores around 100,000 times narrower than a human hair. These tiny pores help trap the bulky polysulfides while still allowing sodium ions to pass through during charging and discharging.
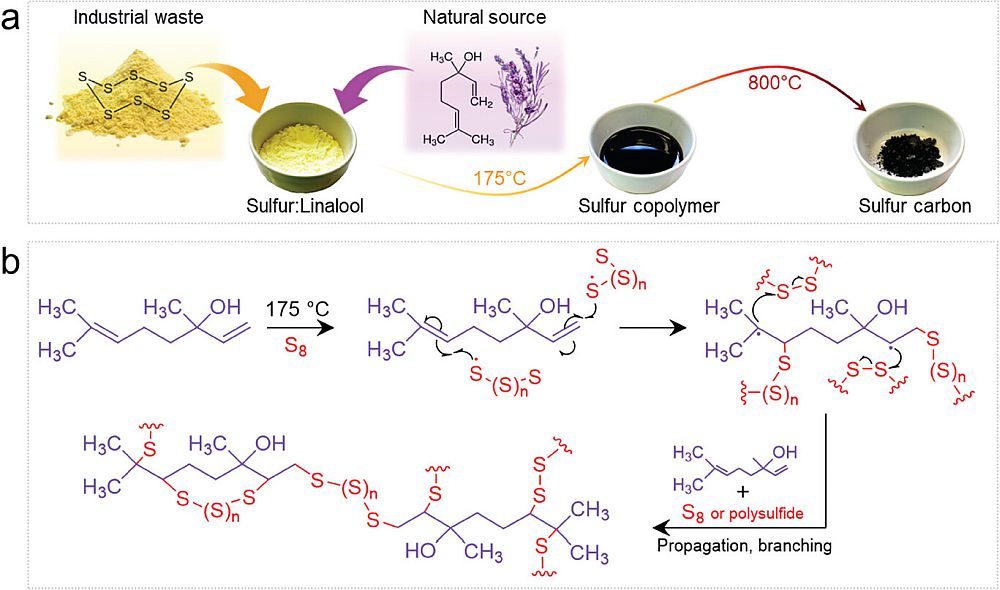
a) Schematic diagram illustrating the synthesis of the hybrid sulfur–carbon nanostructure and b) scheme of the inverse vulcanization reaction of elemental sulfur and linalool. Credit: Senokos et al., Small (CC BY-NC-ND 4.0)
Testing revealed that sodium–sulfur batteries featuring this hybrid nanostructure could retain more than 80% of their original charging capacity (611 mAh/g) after 1,500 charging and discharging cycles. In the paper’s conclusion, the researchers express hope that a range of plant-derived terpenes (aromatic compounds) similar to linalool could be used to produce hybrid nanostructures as well.
“It’s fascinating to design future batteries with something that grows in our gardens,” says senior author Paolo Giusto, group leader of colloid chemistry at the Max Planck Institute of Colloids and Interfaces, in a press release.
The open-access paper, published in Small, is “Sustainable sulfur–carbon hybrids for efficient sulfur redox conversions in nanoconfined spaces” (DOI: 10.1002/smll.202407300).
Author
Lisa McDonald
CTT Categories
- Energy
Related Posts
‘Fairy circles’ may help mark natural underground hydrogen deposits
September 18, 2025


