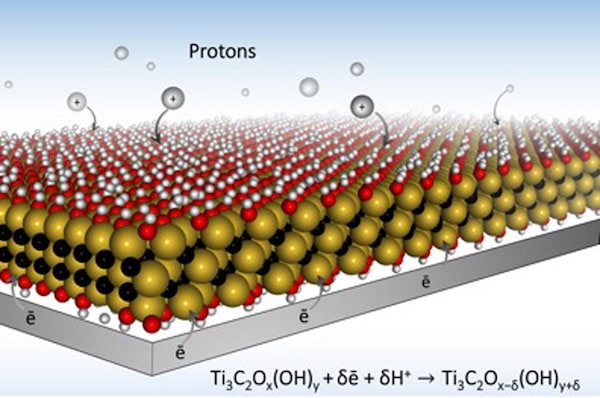
[Image above] Drexel researchers developed electrode designs using MXene that allow for much faster charging because they open up paths for ions to quickly travel within the material. Credit: Drexel University
We love our smartphones, but we don’t love the amount of time it takes to charge them. You have probably been through the frustration of running out of juice when you’re out and about. You pull out your phone for that important call or some other battery-draining activity—like taking pictures or using the GPS—and discover your battery capacity is at 10%. Yikes!
And when you finally do locate a convenient electrical outlet, there you sit, impatiently hovering over the phone for what seems like forever.
Until we get better at planning ahead by charging our phones in advance, we’ll just have to put up with this minor inconvenience. But maybe not for long.
This first-world problem could soon disappear for good, thanks to researchers at Drexel University. A team in the Department of Materials Science and Engineering, led by Yury Gogotsi, Distinguished University and Bach professor at Drexel’s College of Engineering, has developed a new design for a battery electrode that can deliver faster charging capabilities—not just for mobile phones, but for laptops, other electronics, and especially for electric vehicles.
MXenes—highly conductive, ideal for rechargeable batteries
According to a news release on Drexel’s website, the way a battery electrode is designed can determine the speed of charge. The researchers used MXene, a highly conductive material they discovered several years ago, as their electrode material.
MXenes are a group of 2-D transition metal carbides that can be used in energy storage systems and were developed by the A.J. Drexel Nanomaterials Group along with the MAX/MXene Research Group. They function well in other applications, such as shielding our electronics from interfering radio waves, which we reported on last year. But MXenes’ key property is their conductivity, which makes them a perfect material for battery membranes.
Rechargeable batteries need storage capacity—which electrode materials provide in the way of ports, otherwise known as redox active sites. The more of these ports a battery has, the longer the battery will last when charged.
Collaborating on the project were two researchers from Paul Sabatier University (France), who used MXene and a hydrogel combined with oxide metal to design electrodes with more redox active sites than a typical rechargeable battery. The Drexel team added numerous tiny openings in each site to allow for more ions to pass through easily.
“In traditional batteries and supercapacitors, ions have a tortuous path toward charge storage ports, which not only slows down everything, but it also creates a situation where very few ions actually reach their destination at fast charging rates,” Maria Lukatskaya, one of the researchers at the A.J. Drexel Nanomaterials Institute, explains in the news release.
It’s kind of like traveling along the interstate versus taking the back roads if you’re on your way to an important meeting. Given a choice, wouldn’t you take the interstate to get to your destination faster? Ions have the same thing in mind—they want to hook up with electrons as quickly as possible. And when they can travel faster, the battery charges more quickly.
That would solve our slow-charging smartphone problem. But the more important significance of this research is in electric vehicles, where battery charging continues to be an ongoing challenge plaguing researchers.
Gogotsi believes batteries can be improved with better conducting materials. “If we start using low-dimensional and electronically conducting materials as battery electrodes, we can make batteries working much, much faster than today,” he says in the release. “Eventually, appreciation of this fact will lead us to car, laptop, and cell-phone batteries capable of charging at much higher rates—seconds or minutes rather than hours.”
The paper, published in Nature Energy, is “Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides” (DOI: 10.1038/nenergy.2017.105).
Author
Faye Oney
CTT Categories
- Basic Science
- Electronics
- Energy
- Material Innovations
- Nanomaterials


