[Image above] Illustration showing a novel synthesis method for etching tungsten-based MXenes from nanolaminated ternary carbide precursors. Credit: Babak Anasori, Purdue University
Though hydrogen itself is a colorless gas, recent news stories on the anticipated hydrogen economy are filled with references to green, blue, pink, and more colors of hydrogen to distinguish between the many different hydrogen production methods currently being researched.
Though hydrogen hype may feel like a modern phenomenon, excitement over this “fuel of the future” has come and gone in waves over the past five decades. This time, though, some real progress has been made on multiple fronts (albeit slower than originally envisaged).
On the application side, industries such as glass and steel have successfully trialed 100% hydrogen firing in their plants, while hydrogen-powered vehicles such as cars, trains, and planes have taken to the roads and sky. On the storage side, the groundwork has been laid to use different types of underground environments as reservoirs.
But as the plethora of color-coded hydrogen production methods demonstrates, researchers are still grappling with the best way to generate hydrogen in a cost-effective and environmentally friendly manner.
Methods based on electrolysis, or water splitting, are generally considered to be the best option in terms of having the fewest carbon emissions. But these methods are currently much more expensive than fossil fuel-based processes due to the cost of renewable energy and electrolysis technology.
The platinum-based catalysts used to trigger and accelerate the hydrogen evolution reaction are one of the priciest parts of the electrolysis process. Platinum is a rare precious metal that is mined and refined via a complex and costly process. But currently no other more affordable catalyst can match platinum in terms of activity and stability.
Besides finding ways to use platinum more efficiently in electrocatalysts, researchers are working to identify alternative, non-platinum catalysts that can match performance at a lower cost.
MXenes are a novel possibility for next-generation electrocatalysts. This family of 2D transition metal carbides, nitrides, and carbonitrides are known for having excellent electrical conductivity, hydrophilicity, high specific surface area, and desirable electrochemical properties.
Tungsten-based MXenes specifically are predicted to be good electrocatalysts for hydrogen production due to having low overpotential. In other words, the difference between the actual potential required for an electrochemical reaction to occur and the theoretical potential is minimal, meaning the reaction requires less energy to occur efficiently.
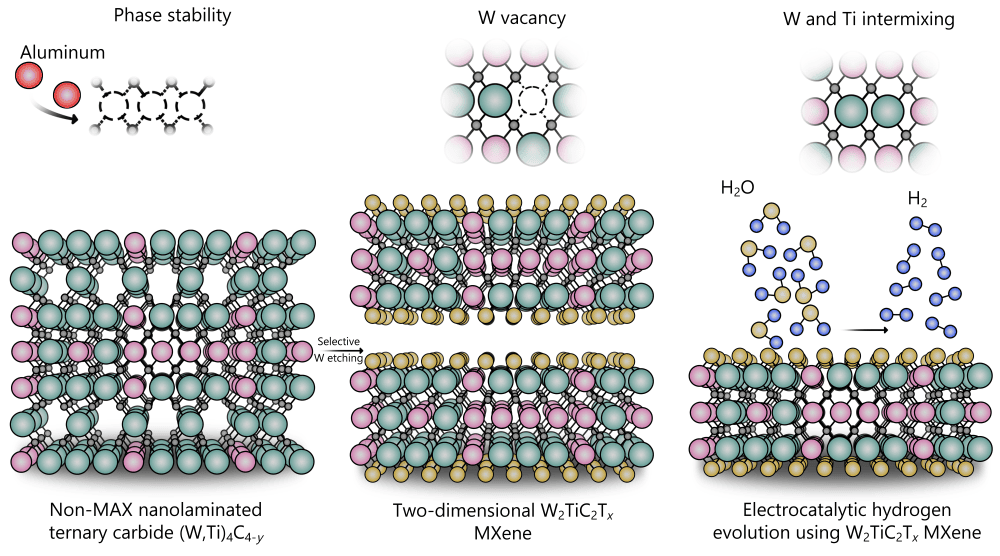
Synthesizing tungsten-based MXenes has proven difficult, however. MXenes traditionally are fabricated by strategically etching away the “A” layer in a precursor MAX phase. But theoretical predictions indicate that tungsten-based MAX phases are inherently unstable. As such, there were no reports of successfully synthesized tungsten-based MXenes in the literature—until last month, that is.
In a March 2025 paper published in Nature Synthesis, researchers led by Babak Anasori of Purdue University reported the successful synthesis of a tungsten-based MXene using a novel processing route. Instead of etching a traditional MAX precursor, which is metallically bonded, the researchers instead etched a nanolaminated ternary carbide precursor, which is covalently bonded.
This study is the first time that a layered carbide consisting entirely of transition metals has been etched, Anasori explains in an email. To accomplish this feat, they first used density functional theory to calculate the energy required for etching each layer. They also investigated the effect of intermixing transition metals—tungsten and titanium—and the effect of vacancies in tungsten atomic layers.
Using the knowledge gained from these calculations, the researchers mixed tungsten, titanium, and carbon together with some aluminum to control the arrangement of tungsten and titanium atoms in each atomic layer. They then etched the mixture using hydrofluoric acid in a process carried out over four days at 55°C. The process yielded a multilayered structure that could then be delaminated to obtain the tungsten-based MXenes.
The tungsten-based MXene with composition W2TiC2Tx had the lowest overpotential (~144 mV), which is about 25% lower than the overpotential (~186 mV) of the previously best performing MXene electrocatalyst (Mo2Nb2C3Tx) reported by Anasori’s group. This value is noticeably closer to the overpotential of platinum, which ranges between 20–40 mV in acidic electrolytes.
While low overpotential alone does not guarantee improved electrocatalytic activity, several other parameters indicated this MXene would perform well as a hydrogen catalyst. Specifically, the MXene had a low Tafel slope, which suggests faster reaction kinetics, and experimentally confirmed stability for about 24 hours in an acidic environment. The researchers attribute these desirable properties to tungsten’s highly active and ordered basal planes.
The success of this new MXene and MXene synthesis method “opens avenues for future research in exploring the synthesis of other non-MAX nanolaminated ternary carbide precursors,” the researchers write.
They expect future studies will reveal many more applications for the tungsten-based MXenes besides just hydrogen production. For example, preliminary analysis of the MXenes in this study revealed desirable electronic and optoelectronic behaviors that could be used for various photonic and optoelectronic applications, such as optical limiting and all-optical switching.
The paper, published in Nature Synthesis, is “Synthesis of a 2D tungsten MXene for electrocatalysis” (DOI: 10.1038/s44160-025-00773-z).
Author
Lisa McDonald
CTT Categories
- Energy
- Manufacturing
- Material Innovations
Related Posts
Sports-quality ice: From pond side to precision Olympic engineering
February 12, 2026
Solid-state batteries turn heads at CES 2026
January 29, 2026


