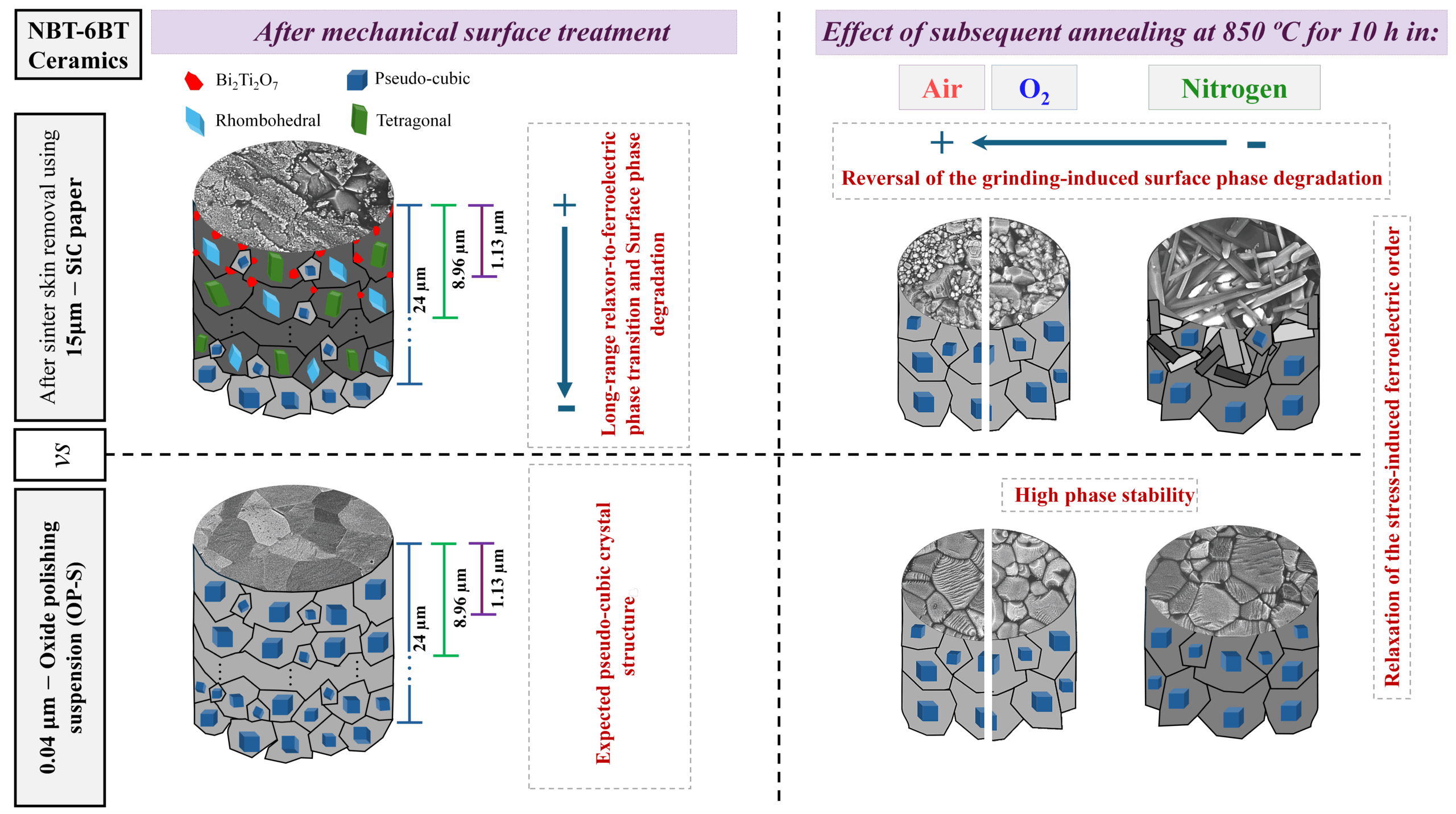
[Image above] Credit: Catalina Olavarria; Flickr CC BY-NC-SA 2.0
There’s somewhere in the neighborhood of 326 million trillion gallons of water cycling around on our planet.
And there’s no dearth of metal oxides either. So it’s important to know the basic principles of how these substances interact with one another—not only because they’re prevalent, but also because they’re used in so many applications vital to our daily lives. Metal oxides are extremely important commercially—they’re used in various forms for catalysts and catalyst supports, as gas sensors, and much more. Sometimes, their commercial importance stems from their unwanted presence, as evidenced by the $3 trillion per decade corrosion problem.
Researchers from Aarhus University, Lund University, and the University of Wisconsin-Madison are collaborating to learn more about how metal oxide surfaces interact with those 326 million trillion gallons of water.
The research, published in Nature Communications, “opens doors for greater understanding and control of chemical reactions in fields ranging from catalysis to geochemistry and atmospheric chemistry,” according to a University of Wisconsin-Madison press release.
In fact, “Ninety percent of all catalytic processes use metal oxides as a support,” Manos Mavrikakis, senior author and Wisconsin engineering professor, says. “Therefore, all of the reactions including water as an impurity or reactant or product would be affected by the insights developed.”
Because metal oxides have occasional “oxygen defects,” or regions where an oxygen atom is missing, scientists previously didn’t understand how water molecules interacted with them in contrast to the interaction with more homogeneous non-oxide metals.
“When water meets with one of those defects, it forms two adjacent hydroxyls—a stable compound comprised of one oxygen atom and one hydrogen atom,” the press release states. But then what?
The researchers probed this knowledge gap, trying to understand how the hydroxyl molecules affected surrounding water molecules. Researchers at Aarhus characterized the reactions with scanning tunneling microscopy, then Wisconsin scientists picked the images atomically apart with quantum mechanical analysis. Their scrutiny found two very different results.
“On a smooth surface, you form amorphous networks of water molecules, whereas on a hydroxylated surface, there are much more structured, well-ordered domains of water molecules,” Mavrikakis says.
On the hydroxylated surfaces, the researchers think that a hydroxyl allows a hexameric ring of water molecules to form around it on the surface.
The work has a variety of applications beyond basic science, too. “It opens the doors to using hydrogen bonds to make surfaces hydrophilic, or attracted to water, and to (template) these surfaces for the selective absorption of other molecules possessing fundamental similarities to water,” Mavrikakis says. “Because catalysis is at the heart of engineering chemical reactions, this is also very fundamental for atomic-scale chemical reaction engineering.”
The paper is “Water clustering on nanostructured iron oxide films” (DOI: 10.1038/ncomms5193).
[Title inspiration credit goes to Sum 41—a harkening back to my early pop-punk days. (If there’s a Weird Al of the materials science world, you can take the title as inspiration for your next big hit!)]
Author
April Gocha
CTT Categories
- Basic Science