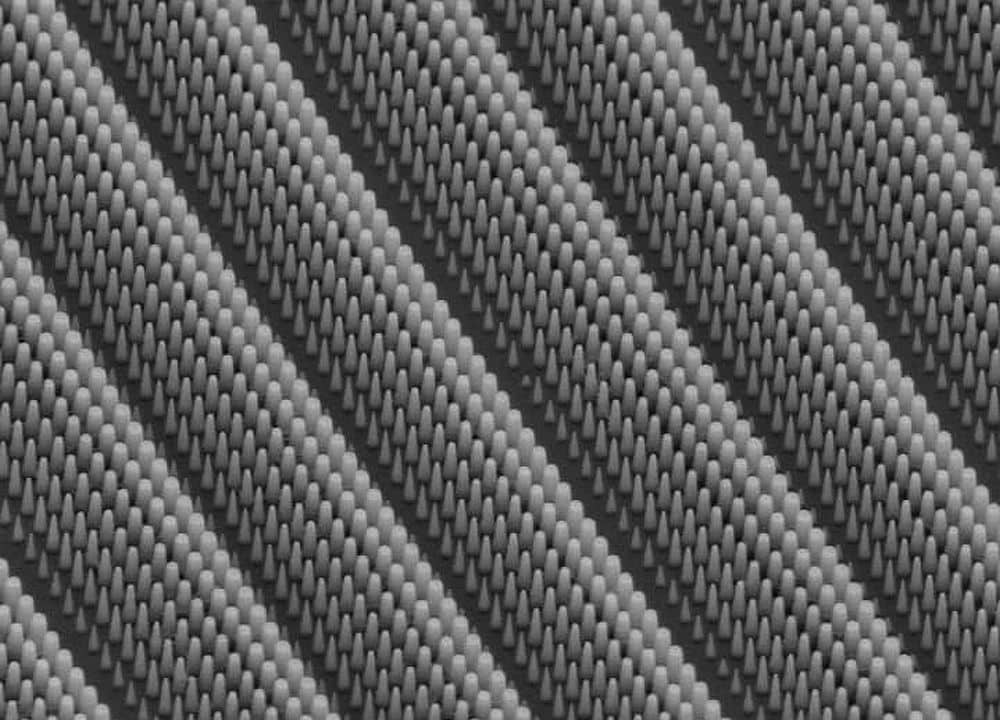
[Image above] Researchers at the University of Central Florida have developed a method to fabricate thin, flexible supercapacitors from 2-D materials. Credit: University of Central Florida
I’m at that dreaded point—I’m nearing, but am still a few weeks shy of, the day when I’m eligible for a new phone from my wireless carrier.
What’s a few weeks, right?
Wrong.
Because my old phone is rapidly biting the dust. Its once-impressive battery can’t hold a charge anymore, so my next few weeks will be filled with anxiety anytime I’m distant from a power outlet. Low battery anxiety is a real thing.
That’s one of the problems with lithium-ion batteries. While they can hold a lot of energy in a relatively compact package, they wear out over time—it’s simple chemistry.
Which is also one reason why supercapacitors are touted as the energy storage solution of our future. While batteries rely on a chemical reaction inside to provide power, supercapacitors instead store energy via separation of electrostatic charges between two electrodes. And without a chemical reaction to wear out over time, supercapacitors can charge and discharge without losing performance for much, much longer—some say 10–20 years.
In addition to a longer life, however, supercapacitors have another major advantage: speed. While conventional batteries store a decent amount of energy, they are slow to charge. Supercapacitors instead can offer up their energy potential almost instantly, allowing the ability to charge devices, such as your smartphone, in seconds.
But although supercapacitors have a lot going for them, the one place they fall short is in energy density—they just don’t have the energy storage potential of lithium-ion batteries.
Yet.
Researchers are searching for new materials that will allow supercapacitors to store sufficient amounts of energy to put them on par with today’s best batteries. Ideal supercapacitor materials need to have high surface area for maximum charge potential, yet also need to be electrically conductive.
We’ve seen instances of supercapacitor concepts made from cigarette butts and carbon films. But one of the most promising possibilities that researchers have been investigating is 2-D materials, whose high surface area and conductive abilities could allow engineers to cram such superior energy storage abilities into a sufficiently small supercapacitor packages.
But despite all the buzz and promise of 2-D materials, incorporating them into supercapacitors and other devices hasn’t proven exactly easy. It’s similar to the story with 2-D supermaterial graphene—there’s been an incredible amount of research on graphene, yet a disproportionately small amount of commercial graphene products to date.
However, 2-D materials may soon get their place in the supercapacitor spotlight, bringing this technology to the forefront of meeting our energy storage and supply needs.
Researchers at the University of Central Florida (Orlando, Fla.) have developed a technique to incorporate 2-D materials into thin and flexible supercapacitor nanostructures that rapidly provide sufficient power and remain stable after countless charging cycles.
“If they were to replace the batteries with these supercapacitors, you could charge your mobile phone in a few seconds and you wouldn’t need to charge it again for over a week,” said Nitin Choudhary, a postdoctoral associate who conducted much of the research, in a UCF news release.
The UCF researchers’ 2-D material of choice is transition metal dichalcogenides, semiconductor materials with a host of properties that make the materials well-suited for various electronic applications.
To incorporate the 2-D materials into supercapacitors, the team devised a technique to coat transition metal dichalcogenide layers onto thin nanowires, creating core-shell structures. The core structures provide conductivity, while the shells yield high energy and power densities—so that the combined structures hold sufficient power yet still charge and discharge rapidly.
“There have been problems in the way people incorporate these 2-D materials into the existing systems—that’s been a bottleneck in the field,” principal investigator and assistant professor Yeonwoong “Eric” Jung says in the news release. “We developed a simple chemical synthesis approach so we can very nicely integrate the existing materials with the 2-D materials.”
An although simple, the team’s synthesis approach is highly effective.
While lithium-ion batteries significantly degrade and thus ultimately fail at less than 1,500 charging cycles, recent supercapacitors have stretched the cycles to a few thousand. But the UCF researchers’ new core-shell supercapacitors can recharge 30,000 times without degrading, according to the news release.
Because this is such a major advance, the team is now working to patent the fabrication process. “It’s not ready for commercialization,” Jung says in the release. “But this is a proof-of-concept demonstration and our studies show there are very high impacts for many technologies.”
The paper, published in ACS Nano, is “High-performance one-body core/shell nanowire supercapacitor enabled by conformal growth of capacitive 2D WS2 layers” (DOI: 10.1021/acsnano.6b06111).
Author
April Gocha
CTT Categories
- Electronics
- Energy
- Material Innovations
- Nanomaterials
Related Posts
Sports-quality ice: From pond side to precision Olympic engineering
February 12, 2026