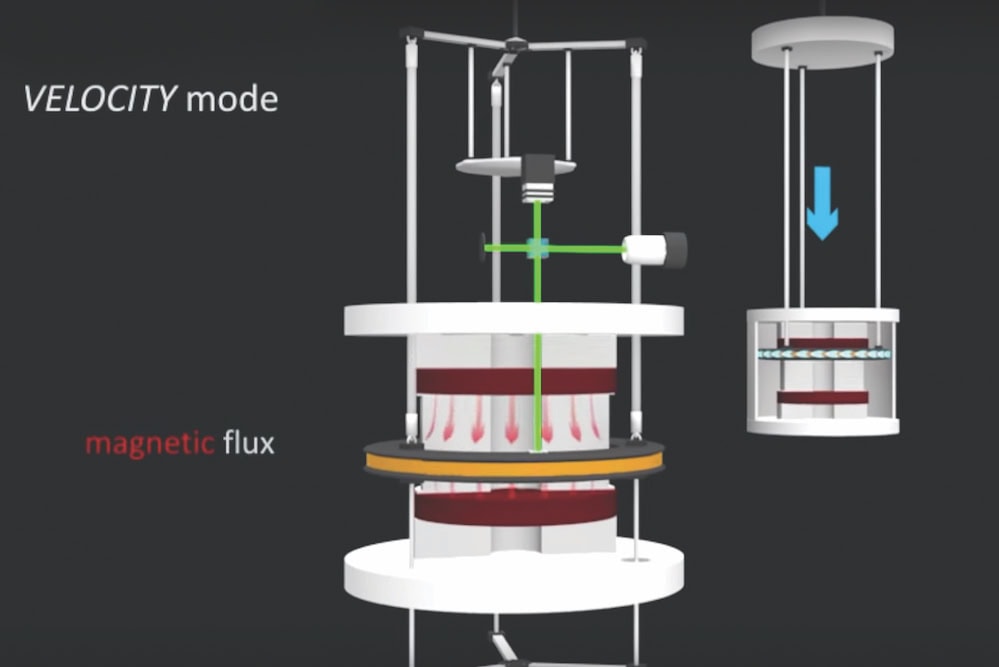
[Image above] NIST researchers used a Kibble balance to measure Planck’s constant, a necessary step in redefining the kilogram. As of November 16, all seven base SI units are defined using fundamental physical constants. Credit: NIST
A truly “mass”ive change took place in the measuring world this month. On November 16, a convocation of delegates representing 60 countries voted to redefine the kilogram based on a fundamental physical constant. With this vote—which also redefined three other basic SI units—all seven base SI units are now defined by fundamental constants rather than physical artefacts.
The International System of Units (SI units)—commonly known as the metric system—is the international standard for measurement. All measurements in the system come from seven base SI units that label seven base quantities assumed to be mutually independent: mass (kilogram); length (meter); time (second); temperature (kelvin); amount of substance (mole); electric current (ampere); and luminous intensity (candela). The November 16 vote redefined four of these units: the kilogram, ampere, kelvin, and mole.

This line graph shows how the base SI units are converted into additional units representing larger or smaller values using powers of 10, a method called unit conversion. Credit: sciencepost, YouTube
There are two ways the seven base SI units are used to create other measurement units: unit conversion, the method for modifying the base units by powers of 10 to more clearly communicate very large or very small values (consider, for example, the centimeter); and derived units that combine base units to create new units, 22 of which have been given special names, like the newton (defined as one kilogram meter per second squared).
But what defines a meter, or any of the other six base SI units?
The French Academy of Sciences created the metric system with the idea to provide a system of measurement derivable from unchanging phenomena so that there would be no question as to the exact value of a measurement. However, finding something that never changes is a difficult task. The metal cylinder previously used to define the kilogram was kept in an environmentally-controlled and vacuum-sealed chamber, but even this carefully kept artefact’s mass changed with respect to sister copies around the world over time. This inherent uncertainty of physical artefacts led scientists to pursue definitions of the base SI units using fundamental physical constants instead.
Fundamental physical constants define the value of certain unchanging physical phenomena in the universe, like the speed of light in a vacuum or the charge of an electron (see a short list and extensive list of all the fundamental constants). Measuring these constants accurately takes advanced technological tools, and only in the last 50 years have scientists reached the point they feel confident redefining the base SI units using these values.
In today’s video, science communicator Derek Muller, creator and host of the YouTube channel Veritasium, visited the National Institutes of Standards and Technology to learn how researchers measured the fundamental Planck’s constant in order to redefine the kilogram (spoiler: it is a very “weighty” video).
Now that all seven base SI units are defined in terms of fundamental constants rather than pesky physical artefacts, the cost of calibrating industrial processes and scientific instruments should go down. While consumers and most industries will likely not notice immediate impacts, the redefinition is a landmark moment for metrologists.
“Using the fundamental constants we observe in nature as a foundation for important concepts such as mass and time means that we have a stable foundation from which to advance our scientific understanding, develop new technologies and address some of society’s greatest challenges.”
Martin Milton, director of the International Bureau of Weights and Measures

Credit: Veritasium, YouTube
Author
Lisa McDonald
CTT Categories
- Basic Science
Related Posts
‘Fairy circles’ may help mark natural underground hydrogen deposits
September 18, 2025


