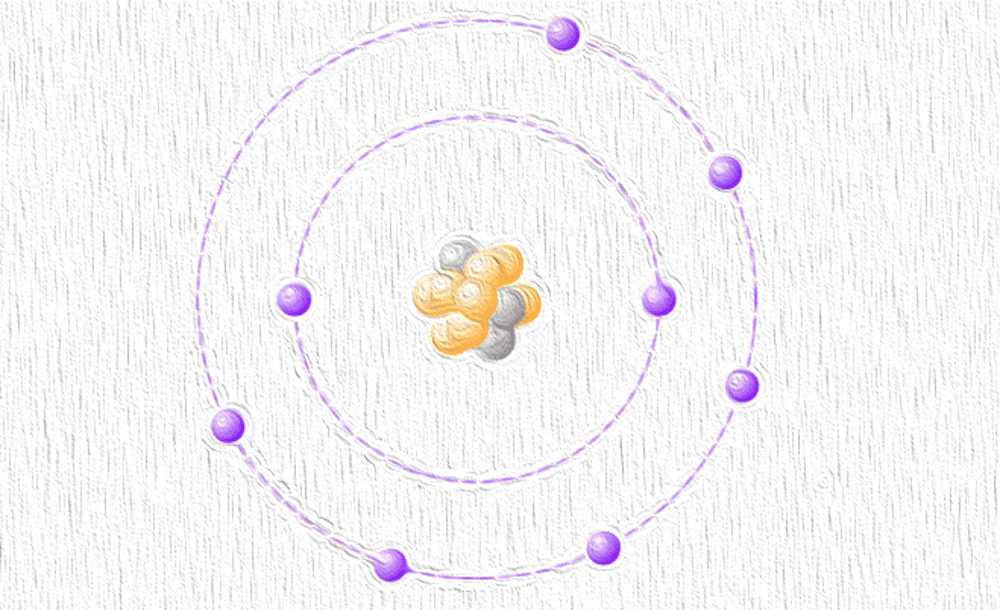
[Image above] Dubbed “featherweight oxygen,” oxygen-11 has only three neutrons to its eight protons, in contrast to eight neutrons in oxygen-16. Credit: ACerS
What is the difference between ions and isotopes?
I frequently ask the chemistry students I tutor this question because the two words can be easily confused. Both words describe variations in the atoms of an element, but while ions describe elements whose atoms come with different numbers of electrons (think Fe2+ and Fe3+), isotopes describe elements whose atoms come with different numbers of neutrons (think carbon-12 and carbon-14).
Ions and isotopes are both highly important concepts in materials science. Among other things, ions determine how atoms will bond together (a molecule’s structure) and isotopes determine an atom’s stability (how likely it is to decay). Knowing the ions and isotopes of a given element helps researchers understand why materials composed of the same element may behave differently from each other (for example, look at the varying colors of transition metal ions in solution).

Both solutions contain iron, but the green one (left) has Fe2+ while the reddish-brown one (right) has Fe3+. Credit: Yeo Yong Kiat, YouTube (Fe2+ and Fe3+)
2019 is the International Year of the Periodic Table, and that means celebrating not just the 118 confirmed elements, but all confirmed ions and isotopes as well. It also means celebrating newly discovered ions and isotopes, like a new oxygen isotope announced last month.
Even though the Washington University press release came out April 1, the researchers’ discovery was certainly no joke. They discovered and characterized oxygen-11, the lightest-ever isotope of oxygen.
Dubbed “featherweight oxygen,” oxygen-11 has only three neutrons to its eight protons. This isotope is noticeably lighter than oxygen-16, which has eight neutrons and is oxygen’s most natural abundant form (about 99.7 percent).
The researchers—from Washington University, Western Michigan University, University of Connecticut, and Michigan State University—created oxygen-11 in the National Superconducting Cyclotron Laboratory (NSCL) at Michigan State University. They performed one- and two-neutron knockout reactions on an oxygen-13 beam to reduce the neutron count from five to three.
While discovering a new isotope is exciting, the researchers have another, greater reason to be excited. “What is most interesting to the nuclear physics community, however, is that oxygen-11 is the nuclear mirror of lithium-11, a very well-studied heavy isotope of lithium,” says Tyler Webb, a Ph.D. candidate in physics at Washington University and lead author of the study, in the press release.
In nuclear physics, a “nuclear mirror” refers to nuclei that have the same mass, but their number of neutrons and protons are reversed. For example, the ratio of neutrons to protons in oxygen-11 is 3:8, compared to the 8:3 ratio in lithium-11. Because protons and neutrons are very nearly identical (the one major difference being protons have a positive charge and neutrons have none), studying mirror nuclei gives scientists great insight into nuclear forces and proton-neutron symmetries.

The researchers’ calculations showed similar orbital motions of the weakly bound particles, whether protons in oxygen-11 or neutrons in lithium-11. Credit: Webb et al., National Superconducting Cyclotron Laboratory
At this point, discovering any new elements beyond the 118 confirmed will be a difficult task. However, the opportunity to create new isotopes offers a vast playing field for scientists to explore. And researching isotopes has a lot to offer to both basic science and applications, according to NSCL laboratory director Brad Sherrill.
“We often talk about nanotechnology and building things on the atomic scale. But we’re really now also in the realm of building things on the nuclear scale,” he says in a video on the importance of isotopes. “… We’re just at the beginning of this [exploration], and as we move into the next generation of facilities that are even more powerful in producing new isotopes, we’ll see another dramatic expansion in our understanding of a variety of things.
The paper, published in Physical Review Letters, is “First observation of unbound 11O, the mirror of the halo nucleus 11Li” (DOI: 10.1103/PhysRevLett.122.122501).
Author
Lisa McDonald
CTT Categories
- Basic Science