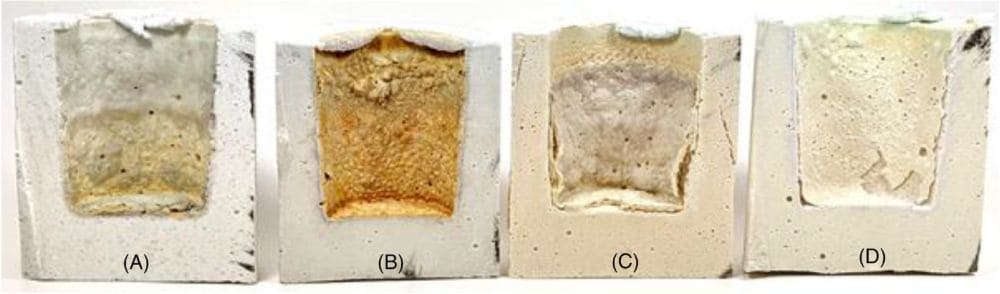
[Image above] Post-test cross-sections of aluminosilicate crucibles that underwent firing with sodium carbonate. Credit: Haines et al., International Journal of Ceramic Engineering & Science (CC BY 4.0)
If you search for “alkali-silica reaction” online, you will find page after page on the deleterious effects of this reaction on concrete. But civil engineering is not the only industrial sector concerned with reactions between alkalis and silica. The refractories industry also faces the challenge of alkali attack on aluminosilicate castables.
Aluminosilicate castables are used extensively in high-temperature industrial applications due to their desirable properties, ease of availability, and cost advantages. However, in many of these applications, the castables are exposed to alkali in the form of vapors and slags. When these alkalis infiltrate the castables’ pores, it can lead to chemical reactions and phase transformations that damage the refractory directly or make it more susceptible to degradation.
There are several ways to reduce the detrimental effects of alkali attacks on refractories, such as by doping the refractory material or reducing the amount of available pore space. To test the effectiveness of these methods, researchers often rely on the crucible cup test (ASTM C987), which replicates an aggressive glass melting furnace environment.
ASTM C987 is a pass/fail test based on visual observation of the refractory after exposure to alkali vapors alone. However, as noted above, aluminosilicate castables are generally exposed to alkalis in various forms, not just vapors. Thus, this test limits a researcher’s ability to determine how a refractory may behave in real-world application.
In a recent open-access paper published in International Journal of Ceramic Engineering & Science, three researchers from Allied Mineral Products in Columbus, Ohio, explored a new procedure that would subject refractories to both visual analysis and formal analytical testing after exposure to alkali slag and vapor.
They created five mix designs for the project, all developed on a volume percent basis to account for differences in specific gravity of the components. Two of the five mix designs were developed with the intent to produce pure mullite (3Al2O3·2SiO2), while two contained zircon within the mix. The fifth mix was alumina-rich relative to the other designs.
They used these mixes to cast crucibles, which were filled with 20 grams of granular anhydrous sodium carbonate and sealed with lids of the same mix designs. The crucibles were then fired using a multistep firing schedule. After firing, the crucibles were divided into quarters using a wet tile saw, and these quarters were used as representative samples for various tests. Crucibles that did not undergo firing with sodium carbonate were also analyzed for comparison.
Four of the five crucibles remained intact after firing with the sodium carbonate; the fifth alumina-rich mix design failed catastrophically and could not be cross-sectioned. Of the crucibles that could be examined, the researchers found their new procedure allowed them to directly relate the different sodium aluminosilicate phases to corrosion of the refractories.
“Additionally, understanding the mechanism of how these phases can form due to accessible porosity, or lack thereof, is crucial when considering the campaign life of refractory to be used in a corrosive environment,” they add.
The open-access paper, published in International Journal of Ceramic Engineering & Science, is “Alkali resistance testing methodology and development: Focus on mullite based castables” (DOI: 10.1002/ces2.10131).
Author
Lisa McDonald
CTT Categories
- Refractories