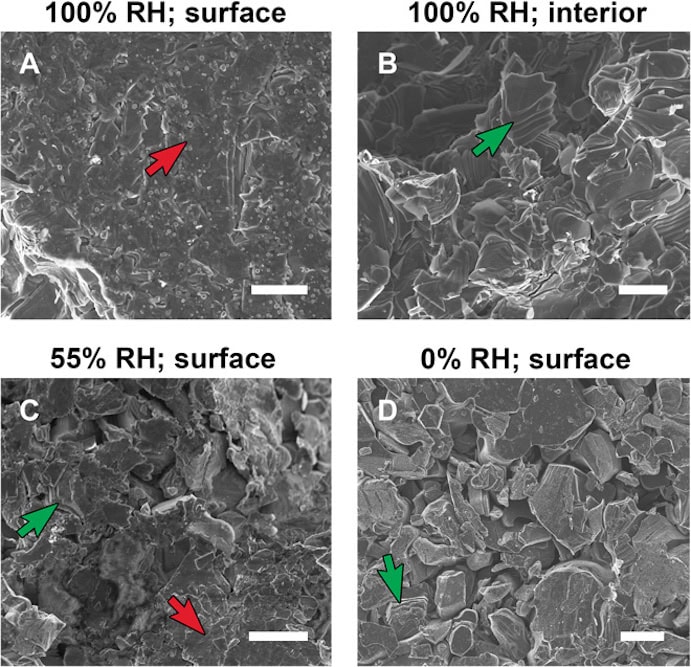
[Image above] SEM images of various pressed MXene discs. Examples of multilayered particles at various states of shear are labeled with green arrows; regions of extreme shear are labeled with red arrows. Credit: Michel Barsoum, Science Advances
When your professor says they need to order “maxenes” before the holidays, likelihood is they do not mean the greeting cards. Instead, your professor is probably preparing to perform research on a family of 2D materials discovered at the start of this decade.
MXenes are a family of 2D transition metal carbides, carbonitrides, and nitrides that were discovered in 2011 by two groups of Drexel University researchers led by Yury Gogotsi and Michel Barsoum, both ACerS fellows and professors of materials science and engineering. MXenes are created by selectively etching MAX phases, which are a large family of ternary compounds consisting of an early transition metal (represented by ‘M’), an A-group element (represented by ‘A’), and a carbon and/or nitrogen (represented by ‘X’). In MXenes, the middle element A is carefully removed from a MAX compound, leaving behind only the M and X (MAX becomes MX). According to Gogotsi’s research webpage, the suffix ‘ene’ was added to emphasize the material’s similarity to graphene.

This periodic table highlights where each of the MAX elements are located: early transition metals (yellow); A-group elements (green); and carbon and/or nitrogen (blue). Credit: Michel Barsoum, Layered Solids Group
MXenes are not a new topic for Ceramic Tech Today—we published multiple articles on MXenes during 2018 (for example, here and here). What is new, though, is the discovery that MXenes expand when compressed in the presence of water.
In a paper by Michel Barsoum and his research group at Drexel University published in January, they found that the MXene titanium carbide (Ti3C2Tx, where T is a variable surface group of -OH, -O, and -F) exhibits pseudo-negative compressibility, a phenomenon typically limited to some clay minerals and carbon-based layered materials. Negative compressibility is when a system increases in size isotropically as hydrostatic pressure (pressure exerted by a fluid) increases, rather than decreasing in size as is typical. The reason negative compressibility occurs is because solvent molecules (water, alcohols, or other organics) insert themselves between the weakly bonded layers of the compressed material, though the exact mechanisms behind the effect have not been perfectly explained to date.
In contrast to negative compressibility, pseudo-negative compressibility is where a system expands along certain directions. In the case of MXene titanium carbide, this layered material expanded along its crystallographic c direction by a large amount when uniaxially compressed into a disc by a steel die and to a small extent when compressed quasi-hydrostatically in a diamond anvil cell. In an email from Barsoum, he explains the compression was quasi-hydrostatic instead of fully hydrostatic because “Water is not a fantastic transducer for perfect hydrostatic pressure in these experiments, and we had more of a slurry, so it is possible we did not have enough fluid to fully say that it was hydrostatic.”
In the paper, Barsoum and his group explain the difference in expansion between the two compression methods is due to shearing. Under hydrostatic compression, pressure comes from all directions, compressing the MXene uniformly on all sides. But when the MXene is compressed uniaxially, the layers are able to slide against each other. “Imagine when you have these multilayers that there are bottlenecks around them that will not let water go in,” says Barsoum in a Drexel press release. “As soon as you shear them, you break that barrier and water flows in and pushes the layers apart.”
Why does it matter that MXenes exhibit pseudo-negative compressibility? One practical concern is in the area of electrical conductivity. In the paper, Barsoum and his group explain that a common way to report MXene conductivity is by first pressing the material into a disc—the compression technique shown to result in a larger expansion. Due to MXenes’ hydrophilic nature (its tendency to mix with water), Barsoum and his group emphasize that humidity should be considered during disc compression, because high humidity in the compression environment could exacerbate the insertion of the water bilayer and lead to changes in the material’s conductivity.
In an email from Barsoum, he says they are not currently doing any more experiments on the expansion aspect of MXene, but there are several other aspects they are looking at. “We are more focused on what happens during etching and more importantly how MXene are [similar] or are not similar to clays,” Barsoum says.
The paper, published in Science Advances, is “Pressure-induced shear and interlayer expansion in Ti3C2MXene in the presence of water” (DOI: 10.1126/sciadv.aao6850).
Author
Lisa McDonald
CTT Categories
- Basic Science