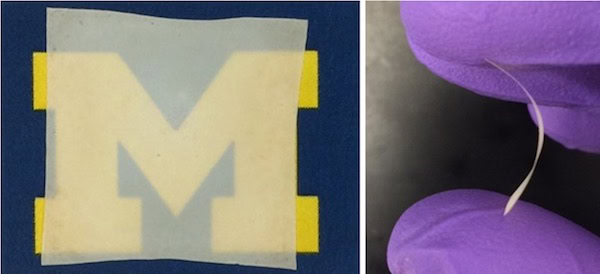
[Image above] A transparent and flexible thin film of LLZO produced by scientists at the University of Michigan. The film is 2 cm x 2 cm. Credit: Laine lab; University of Michigan
While lithium-ion batteries have come a long way, these energy powerhouses aren’t perfect.
Users of Samsung’s explosive Galaxy Note 7 and electronic cigarette smokers can attest to some of those safety-related imperfections.
In addition to improving the safety of lithium-ions, we’re constantly wanting them to do more in terms of performance—more power in a smaller, safer package. So new materials are needed to push the ubiquitous lithium-ion to safer and greater energy storage capacities.
Quick basic battery primer—batteries consist of two electrodes, an anode and a cathode, separated by an electrolyte buffer. Charging and discharging happens when ions move between the electrodes, through the electrolyte.
Most everyday batteries use a liquid electrolyte, partially because the liquid offers reduced resistance for the movement of those ions. But liquid electrolytes have their drawbacks—they have serious safety issues (see above), limit incorporation of other battery materials (compatible chemistries required), and have significant thermal drawbacks (protect your battery from extreme temperatures!).
Solid state batteries—which, as the name might imply, ditch the liquid for a solid electrolyte—can help solve some of the problems that plague batteries with liquid electrolytes. For one, solid state batteries are much safer and have the capacity to offer higher energy densities in smaller footprints.
But researchers are still searching for just the right solid electrolyte materials that can properly perform in solid state batteries.
“Collaborative research is needed to realize all-solid-state lithium batteries due to the interdisciplinary nature of the challenge this technology is faced with,” says Richard Laine, professor of materials science and engineering at the University of Michigan. Laine and his research lab are developing new approaches to process materials for solid state batteries, in addition to a variety of other materials research (such as sustainable approaches for silica production).
Laine, an ACerS member, can attest to the power of ceramic materials as a lithium-ion electrolyte option—ceramics have the potential to eliminate problems with compatible chemistries, offer a safer and more thermally stable solution, and can reduce the size of such batteries, too. In particular, one candidate ceramic is a garnet material called LLZO, which has appeared on CTT before.
“For many years, LLZO has been considered the most promising solid electrolyte in the construction of all-solid-state lithium batteries using lithium metal anodes, which potentially offer higher energy density, longer cycle life, and inherent safety. Fundamental understanding of LLZO has matured over time, whereas processing has fallen short,” Eongyu Yi, a graduate student in Laine’s lab, writes in an email.
An ideal ceramic electrolyte needs to have good ionic conductivity and have wide electrochemical and thermal operational windows, according to Yi. And LLZO does just that.
But an ideal ceramic electrolyte also needs to be able to be formed into thin films using low-cost, mass-producible methods, a must for commercial scale-up—and that’s where LLZO falls short.
That’s because processing the material into thin films requires prolonged sintering at high temperatures (i.e., energy- and time-intensive), which volatize lithium out of the thin film.
So Laine’s research group devised a strategy to process LLZO thin films sans the prolonged sintering route.
The team’s work, published in Journal of Materials Chemistry A, describes how the scientists used flame spray pyrolysis and conventional casting–sintering—proven methods that work on a mass production scale—to fabricate thin films of LLZO. Yi is first author of the new paper.
Using liquid-feed spray pyrolysis (LF-FSP)—a technique the scientists previously developed to process nanoscale ceramic powders—the team first produced Li6.25Al0.25La3Zr2O12 LLZO nanopowders containing 50 wt% excess lithium. That excess lithium helps to balance loss of the element through volatization during sintering.
Then, the scientists ball-milled the nanopowders to a uniformly small size and cast them, quickly sintering them for just an hour (in comparison to conventional sintering times of 10–40 hours) to densify the final thin films.
The scientists’ relatively simple and rapid technique produced LLZO films just 20–30 µm thick—which the authors note is similar to the thickness of commercial polymer separators—that were dense despite their thinness, with fine grain structures.
Grain structure is an important feature for a solid electrolyte because grain boundaries impact the materials’ resistance, with greater resistance inhibiting ion flow and thus battery performance.
In addition, the team’s cast and sintered LLZO thin films were translucent and relatively flexible, meaning that the films could help develop thin and flexible batteries.
Electronics company Panasonic recently unveiled its own flexible, ultra-thin lithium-ion battery, which the company touts can offer improved safety and the ability to remain functional even after repeated bending.
But, according to Yi, Panasonic’s battery likely uses a polymer electrolyte to achieve such thinness, safety, and flexibility. And even though the Panasonic battery touts improved safety, if offers much lower energy density at the same cell voltage of conventional smartphone batteries (3.8 V).
LLZO can do better.
By comparison, LLZO offers a wider window of electrochemical stability than polymers. So LLZO electrolytes can provide 10–100 times higher ionic conductivities than polymers, allowing faster charge and discharge rates at the same battery thickness. Plus, ceramic electrolytes are stable up to high temperatures (>800ºC), whereas polymers can melt above just 60ºC, according to Yi.
“Our results are a step forward to the realization of all-solid state lithium batteries. However, there are more steps to take,” Yi says.
Those steps include reducing LLZO film thickness, sintering temperature, and excess lithium content. “Reduced sintering temperatures will retard Li2O loss rates, widening the optimal processing window,” Yi explains via email. The authors also report in the paper that they’re exploring gallium-doped LLZO thin films as well.
Another important challenge in developing solid-state batteries lies at the interface between the electrodes and electrolytes. Yi explains that the interphase must be electrochemically active for solid-state batteries to perform in optimal conditions. “This is the next challenge the research community will have to solve.”

The interphase area is the solid-solid interface and is an important component of solid-state battery performance and can be formed in situ or ex situ. Credit: Laine lab; University of Michigan
Ultimately, the University of Michigan scientists’ work could help open the door to put LLZO ceramics into solid-state batteries with unprecedented energy storage in very thin, yet very safe, packages.
In the meantime, some manufacturers are already making the move to incorporate ceramic materials into safer lithium-ion batteries. So soon enough, you may no longer have to worry (as much) about your smartphone exploding in your face.
The paper, published in Journal of Materials Chemistry A, is “Flame made nanoparticles permit processing of dense, flexible, Li+ conducting ceramic electrolyte thin films of cubic-Li7La3Zr2O12 (c-LLZO)” (DOI: 10.1039/C6TA04492A).
Author
April Gocha
CTT Categories
- Electronics
- Energy
- Material Innovations
- Nanomaterials


