[Image above] A prototype of the oxygen-ion battery developed by researchers at TU Wien. Credit: TU Wien
With global energy consumption on an ever-upward trajectory, transforming the way energy is collected, stored, and used is necessary to avoid the related rise in carbon dioxide emissions.
Batteries will play an essential role in this transition by allowing energy from renewable sources to be stored long term. However, lithium-ion batteries, which are the keystone of today’s battery market, have several shortcomings, particularly regarding safety. New battery technologies must be developed, then, to meet this demand in a safe, sustainable, and economic manner.
Solid-state batteries are one emerging technology attracting international interest. These batteries use a solid electrolyte rather than the liquid or polymer gel electrolytes found in conventional batteries. Not only does this solid electrolyte prevent thermal runaway and combustion, but it also typically leads to a battery with higher energy density.
Many solid-state batteries under investigated, both in the United States and elsewhere, are based on lithium metal. However, reliance on lithium—which is experiencing shortages and subsequent price hikes due to rising demand for energy storage technologies—could slow battery development.
Instead, researchers are exploring alternative solid-state battery compositions, such as sodium batteries. But so far, all explored technologies face challenges, such as low ionic conductivities or dendrite growth, that hinder their commercialization.
In January 2023, three researchers from the Vienna University of Technology (TU Wien) in Austria announced a novel solid-state battery composition that may offer certain advantages over other battery technologies—oxygen-ion batteries.
In the open-access paper on this discovery, the researchers explain that ceramics called mixed ionic–electronic conducting (MIEC) oxides have the ability to conduct both ions and electrons. This ability allows for the incorporation and evolution of oxygen in a manner similar to how lithium-ion batteries store electrical energy by changing their lithium content.
Despite this similarity, as well as extensive investigations of MIEC oxides as electrode materials in solid oxide fuel cells and solid oxide electrolysis cells, “Systematic investigation of such oxygen insertion electrodes … [to realize] all-solid-state oxygen-ion batteries are not available, to the best of our knowledge,” the researchers write.
To test this potential, the researchers fabricated model cells featuring yttria-stabilized zirconia single crystal electrolytes and several different MIEC thin film electrodes. Specifically, the perovskite-type oxides La0.6Sr0.4FeO3−δ (LSF), La0.5Sr0.5Cr0.2Mn0.8O3−δ (LSCrMn), and La0.9Sr0.1CrO3−δ (LSCr). After conducting half-cell measurements on these different electrodes, they constructed a full oxide-ion battery with an LSF cathode and LSCrMn anode.
DC measurements on the full cell showed capacities as high as 120 mA h cm−3 (normalized to the electrode volume) at a cell voltage of 0.6 V at 350–400°C. The cell exhibited excellent cycling performance as well, with less than 1% of the charge being lost per cycle.
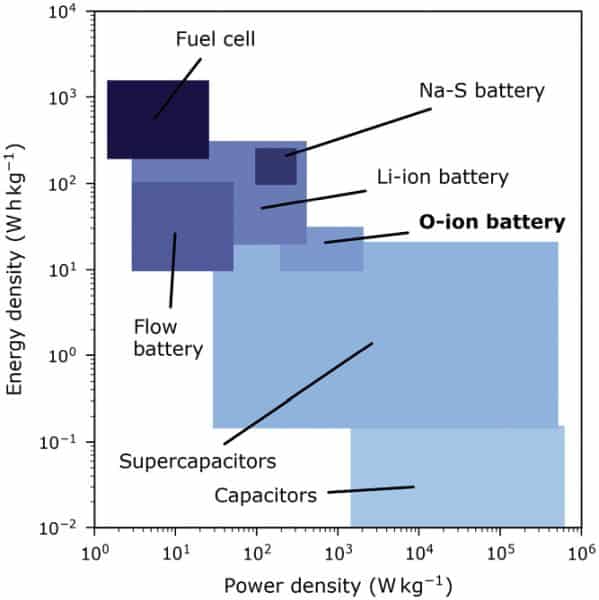
Compared to lithium-ion batteries, the oxygen-ion battery only achieved about a third of the energy density, plus required increased operating temperatures to run (200–400°C). However, neither of these factors play a decisive role in large energy storage applications, and so oxygen-ion batteries could be an “excellent” fit for this purpose, a TU Wien press release reports.
In the press release, first author Alexander Schmid, post-doc in the Institute for Chemical Technologies and Analytics at TU Wien, adds that the oxygen-ion batteries demonstrate another unique advantage—the potential for regeneration.
“In many batteries, you have the problem that at some point the charge carriers can no longer move,” he says. “Then they can no longer be used to generate electricity, the capacity of the battery decreases.”
The oxygen-ion battery, though, can be easily regenerated. If oxygen is lost due to side reactions, it can be compensated for by oxygen from the ambient air.
The researchers have filed a patent application for the oxygen-ion battery together with cooperation partners from Spain. They are now investigating alternative electrode compositions that do not use the rare earth element lanthanum.
The open-access paper, published in Advanced Energy Materials, is “Rechargeable oxide ion batteries based on mixed conducting oxide electrodes” (DOI: 10.1002/aenm.202203789).
