[Image above] Dye-sensitized solar cells use titanium dioxide, but even more solar energy conversion technologies could use TiO2 if an easy way to dope the material with nitrogen is found. Credit: NurdRage, YouTube
With silver bells and holly wreaths decorating the halls, it can only mean one thing—time to apply sunscreen!
Though many equate sunscreen with the beach, winter is also a burn-risk season—snow reflects up to 80 percent UV radiation, much more than the approximately 15 percent reflected by dry beach sand.
As you reach for your sunscreen, take a moment to notice one of the main ingredients: titanium dioxide.
Titanium dioxide (TiO2) is a photocatalyst, meaning it is a material that hosts a chemical reaction after absorbing light. In sunscreen, TiO2 will absorb the incoming UV rays and keep it from reaching your skin, later releasing the absorbed light’s energy as heat. TiO2 is also used in self-cleaning concrete and glass because the absorbed UV light energy breaks down dirt.
Yet despite the myriad uses for TiO2 that scientists found based on the material’s UV ray absorption ability, they are still looking to achieve more—by extending the abilities of TiO2 into the visible light spectrum.
Visible light accounts for about 47 percent of the solar spectrum, while UV light is only 2 percent (infra-red accounts for 51 percent). TiO2 cannot absorb visible light because its band gap is too wide—approximately 3.2 eV, compared to visible light’s range of 1.8-3.1 eV. If the band gap of TiO2 could be narrowed into the range of visible light (in other words, if TiO2 could absorb visible light), its other properties—nontoxicity, high stability, and low cost—would make TiO2 an ideal candidate for green energy technologies, like solar cells.
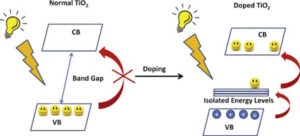
Doping titanium dioxide with different metals and nonmetals narrows the band gap, making the material able to absorb visible light. Credit: Suresh C. Pillai et al., Journal of Photochemistry and Photobiology C
To achieve visible light absorption, scientists have tried doping TiO2 with both metals and nonmetals; specifically, nitrogen showed to be a good doping choice due to its small ionization energy, high stability, and low cost. Several approaches to doping TiO2 nanotube arrays with nitrogen include one-step direct electrochemical anodization of a titanium nitride alloy and hydrothermal reaction. But new research shows an easy way to dope: simple thermal annealing.
A team of Vietnamese and Taiwanese researchers led by Jihperng Leu, professor of materials science and engineering at National Chiao Tung University (Hsinchu, Taiwan), published a recent paper describing how they successfully fabricated nitrogen-doped TiO2 nanotube arrays by annealing the arrays in ambient N2 gas at 450°C (840°F) for three hours. The N-doping concentration (between zero and 9.47 percent) was varied by controlling N2 gas flow rates between 0 and 500 cc/min.
The researchers determined the band gap narrowed noticeably the more nitrogen they deposited, from 3.13 eV (zero percent nitrogen) to 2.95 eV (9.47 percent nitrogen). When they tested the reaction rate, they discovered the TiO2 nanotube arrays doped with 9.47 percent nitrogen had a reaction rate 125 percent higher than that of undoped samples.
“The results of this study demonstrate that the simple annealing process is beneficial for introducing N-doping into TiO2,” say the researchers in the paper.
Titanium dioxide is already commonly used in dye-sensitized solar cells, but with more research like this study, TiO2 may soon be used in far more photovoltaic technologies.
The paper, published in Micromachines, is “Enhanced Photocatalytic Performance of Nitrogen-Doped TiO2 Nanotube Arrays Using a Simple Annealing Process” (DOI: 10.3390/mi9120618).
