
[Image above] Artistic depiction of the rhombic dodecahedron shape that lithium atoms formed when solid electrolyte interphase formation was avoided. Credit: Li Lab, UCLA
Lithium may be the lightest of metals, but it is a heavyweight on the global market. Demand for the little element has skyrocketed with burgeoning demand for portable energy, and governments and companies around the world are concerned about having access to enough supply.
Yet even with the ubiquity of lithium-based batteries in today’s world, there are still aspects of the material that remain elusive. For example, determining the true structure of metallic lithium.
Lithium-metal batteries use metallic lithium as the negative electrode. This electrode is produced using electrodeposition, or a process that uses electric current to reduce a salt solution of the metal to be deposited. During this process, a solid electrolyte interphase (SEI) layer simultaneously forms as well and influences the morphology of the deposited lithium.
The complex feedback loop of SEI formation and lithium deposition is difficult to decouple. As such, researchers have struggled to develop a general framework for understanding and predicting lithium morphology.
Instead, “most descriptions of the structure is qualitative, such as ‘chunky’ or ‘column-like,’” says Yuzhang Li, assistant professor of chemical and biomolecular engineering at the University of California, Los Angeles, in a UCLA press release.
Li is a member of the California NanoSystems Institute at UCLA. In a recent study, he and other members of the institute aimed to modify the electrodeposition process to decouple lithium deposition from SEI growth and thus reveal the intrinsic deposition morphology of metallic lithium.
They hypothesized that the reaction that causes SEI formation could be outpaced if lithium was deposited more quickly by increasing current density. While this premise seems counterintuitive, as previous studies showed damaging dendrite growth is accelerated at higher current densities, “it aligns with expectations if lithium deposition truly proceeds independently from SEI formation,” they write.
The researchers achieved a high current density by running the current through a very small electrode, which allowed electricity to be pushed out faster. They also adjusted the shape of the electrode to sustain ultrafast currents without sudden ion depletion. They then used this high-density and ultrafast current to deposit metallic lithium on four model electrolytes with different chemistries.
When lithium was deposited at typical current densities, the researchers observed distinct lithium morphologies in each model electrolyte (i.e., filaments, rods, columns, and chunks). But when high current density was used, they discovered all four electrolytes demonstrated a stark morphological transition to well-defined faceted lithium polyhedra, specifically rhombic dodecahedra (12-sided figures).
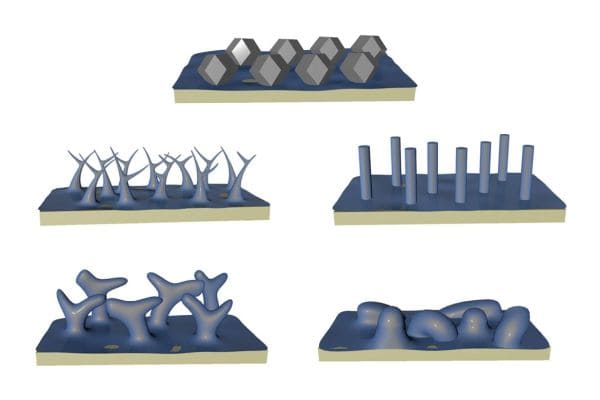
This result suggests three important findings.
- The morphology dependence on electrolyte chemistry disappears at high current densities.
- Lithium deposition and SEI formation can be decoupled at high current densities.
- The faceted polyhedra are the intrinsic deposition morphology of metallic lithium in the absence of dendrite formation.
“Now that we know the shape of lithium, the question is, ‘Can we tune it so that it forms cubes, which can be packed in densely to increase both the safety and performance of batteries?’,” Li says in the press release.
Tuning this technique will require the researchers to identify the minimum current density needed to outpace SEI formation. This minimum will depend on various parameters, including cell geometry, temperature, pressure, electrolyte chemistry, and other factors.
The paper, published in Nature, is “Ultrafast deposition of faceted lithium polyhedra by outpacing SEI formation” (DOI: 10.1038/s41586-023-06235-w).
