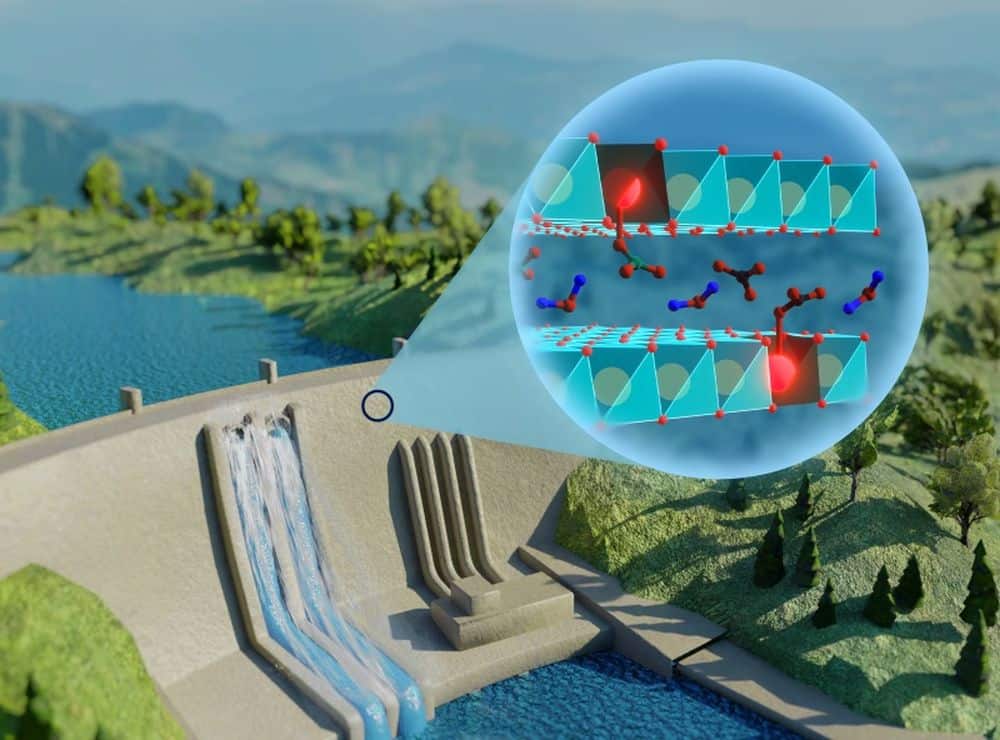
[Image above] Researchers led by the University of São Paulo’s Physics Institute developed a luminescent material that can indicate the degree of carbonation in concrete structures. This image illustrates the absorption and interaction of carbonates with europium in the concrete structure. Credit: Danilo Mustafa, Chemical Communications (CC BY 3.0)
Since the invention of reinforced concrete in the mid-19th century, the use of concrete in construction exploded, leading this material to become the backbone of modern infrastructure.
But with the increasing usage of concrete comes an increasing need for cost-effective and rapid monitoring techniques to ensure the safety of concrete structures. There can be severe consequences if these maintenance needs are not met, as the United Kingdom discovered when more than 100 schools had to shut down or close off areas only days before the new school year started in August 2023.
Corrosion of the reinforcing bar, or rebar, in reinforced concrete is one common cause of concrete deterioration. When reinforced concrete is first poured, the high alkalinity of concrete causes a passivating oxide layer to form on the rebar, protecting it from corrosion. But as carbon dioxide in the air is absorbed into the concrete, it triggers a chemical reaction called carbonation that lowers the pH of the concrete.
This increasingly acidic environment, in addition to the intrusion of chloride ions (present in deicing salts and seawater), cause the passivating oxide layer to break down. Without this protective layer, the rebar is susceptible to corrosion when exposed to moisture and oxygen.
The degree of carbonation in a concrete structure is typically determined by removing a small piece of the structure and then spraying it with a phenolphthalein solution. The phenolphthalein turns bright pink when in contact with pristine concrete, but its color fades or completely disappears when carbonation has occurred.
This procedure is detrimental to the concrete structure because it requires drilling holes in the composite, affecting its structural integrity. A method for determining the degree of carbonation without destroying the structure is preferable because the concrete could then be monitored without affecting its mechanical properties.
In a recent open-access paper, researchers at the University of São Paulo’s Physics Institute in Brazil, in collaboration with colleagues at the University of Leuven in Belgium and the University of Kiel in Germany, developed a luminescent material that can be incorporated into concrete structures. When exposed to ultraviolet light, the luminescent material can indicate the degree of carbonation based on its color.
The luminescent material is a europium-doped zinc aluminum layered double hydroxide (LDH). LDHs, also called anionic clays, are an emerging layered material class that hold great promise as anion exchangers, adsorbents, catalysts, and biomaterials due to their high variability in cation and anion composition.
LDHs are reported to improve the durability of concrete when mixed with the cement. In other words, LDHs can be incorporated directly into concrete structures as a new class of additives.
In this study, the researchers used zinc aluminum LDH as a host material for europium ions, which have photoluminescence properties. When the LDH absorbs carbonates, the materials that result from the carbonation of concrete, it causes a change in the europium coordination number. As more carbonates are absorbed, the luminescence of the europium ions under ultraviolet light shifts from orange to red.
A São Paulo Research Foundation press release reports that the researchers are now developing a sensor that detects the luminescent material within concrete structures. They will then test the luminescent material under real-world conditions to verify its weatherability and stability inside concrete.
The open-access paper, published in Chemical Communications, is “Eu3+ doped ZnAl layered double hydroxides as calibrationless, fluorescent sensors for carbonate” (DOI: 10.1039/D3CC03066K).
