
[Image above] John B. Goodenough in his office at the University of Texas at Austin. Goodenough spent the last three decades of his research career at UT Austin, where he held the Virginia H. Cockrell Centennial Chair in Engineering. Credit: The University of Texas at Austin
As a child of the ’90s, I have never known a world that didn’t rely on lithium-ion batteries. This technology threads through all aspects of my daily life, and I often take it as much for granted as the air I breathe.
When three researchers received the 2019 Nobel Prize in Chemistry for their contributions to the development of lithium-ion batteries, I was shocked to realize how new this technology is. The world’s first commercial rechargeable lithium-ion battery hit the market in only 1991!
This Nobel Prize announcement is also when I learned about John B. Goodenough, the materials scientist and solid-state physicist who showed that cobalt oxides could be used as the cathode in lithium-ion batteries. His cathode was used in those very first rechargeable batteries that Sony released in 1991, and it is still used in batteries today.
CTT published a profile of Goodenough’s early life and development of battery cathodes in 2019 following the Nobel Prize announcement. When I heard that Goodenough died this past Sunday, June 25, at the age of 100, the outpouring of articles reflecting on his memory made me aware of just how much he contributed to science beyond the seminal battery discovery in the late 1970s. For instance,
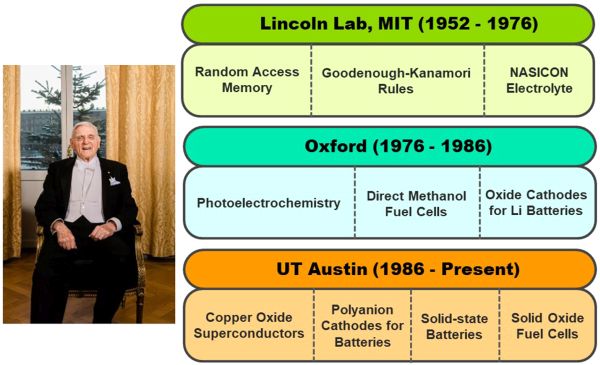
- He made significant contributions to the development of random-access memory (RAM) for digital computers by helping to develop ceramic magnetic memory cores during his time as research scientist and group leader at the Massachusetts Institute of Technology Lincoln Laboratory.
- His research efforts on RAM led him to develop the concepts of cooperative orbital ordering, or cooperative Jahn–Teller distortion, in oxide materials.
- This finding led him to develop rules for the sign of the magnetic superexchange in materials. (Now known as the Goodenough–Kanamori rule due to subsequent tweaks by physicist Junjiro Kanamori.) This rule helped rationalize the magnetic properties of a wide range of materials on a qualitative level.
- He led work on identifying several framework structures that supported fast sodium-ion conductivity, including the well-known NASICON (sodium [Na] Super Ionic CONductor). These structures are a promising solid electrolyte for solid-state sodium batteries.
- He led work on identifying various promising electrode and electrolyte materials for solid oxide fuel cells, including double perovskites.
- He wrote two landmark books on solid-state chemistry: Magnetism and the chemical bond (1963) and, in French, Les oxydes des metaux de transition, or Metallic oxides (1973).
Unsurprisingly, Goodenough won many awards beyond the Nobel Prize in Chemistry, including the U.S. National Medal of Science (2011), the Stark Draper prize (2014), and the Enrico Fermi award (2009). Coincidentally, Goodenough studied under Nobel laureate Enrico Fermi and John A. Simpson, both of whom worked on the Manhattan Project, during his graduate studies at the University of Chicago.
In a University of Chicago news release following his Nobel Prize win, Goodenough talked about how the need for sustainable energy sources inspired much of his research.
“We have to, in the near future, make a transition from our dependence on fossil fuels to a dependence on clean energy,” he said. “So that’s what I’m currently trying to do before I die”—to leave behind a cleaner, better world.
Yet the man behind these research accomplishments is what everyone remembers most. As a University of Texas at Austin news release explains, “Goodenough’s quick wit and infectious laugh were defining characteristics that influenced the level of fame he received. That laugh could be heard reverberating through UT engineering buildings—you knew when Goodenough was on your floor, and you couldn’t help but smile at the thought of running into him.”
Even as we mourn the loss of this brilliant yet humble researcher, educator, and inventor, his memory and contributions to science will continue to light up the imagination of researchers in the future. We will miss you, John.
The New York Times published a heartfelt obituary chronicling Goodenough’s life. A compilation of Goodenough’s extensive publications is available on Semantic Scholar. Of those many papers, six were published in ACerS’ Journal of the American Ceramic Society, which can be seen here.
