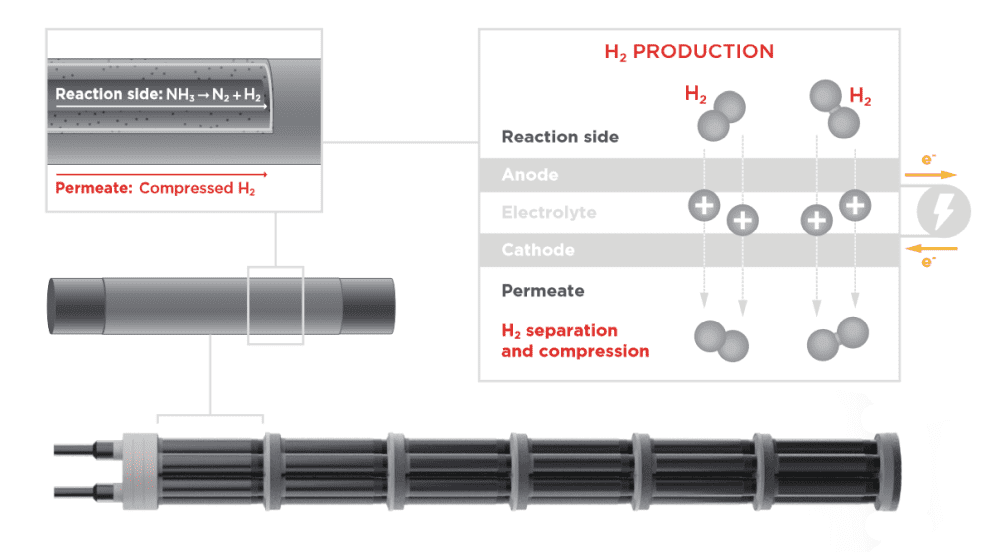
[Image above] Illustration of the new electrochemical membrane reactor designed by CoorsTek-led researchers. It achieved more than 99% hydrogen extraction efficacy. Credit: CoorsTek
Despite aftershocks from the pandemic and a rise in global commodity prices, renewables experienced yet another year of record growth in power capacity in 2021, according to the latest “Renewables Global Status Report” by REN21. But for this growth to continue in 2022, we must further develop the infrastructure necessary for storing generated electricity.
While batteries do well storing energy on an hours timescale, they are less ideally suited to fulfill storage needs on a days to months timescale. Instead, converting electrical energy into a chemical form better serves this purpose.
Hydrogen is one possible long-term storage solution that is attracting much attention. Hydrogen can be easily formed from water via electrolysis, a process that is carbon-free if it uses electricity from renewable energy sources. It can then be burned directly or converted back into electricity, processes in which water is the only byproduct.
However, difficulties associated with transporting hydrogen fuel limit widespread adoption of this technology. Hydrogen is flammable, has a low volumetric energy density, and is easily dispersed into the air. Thus, either high-pressure or low-temperature environments are required to store hydrogen as a gas or liquid, respectively.
Chemical storage offers a potentially easier way to transport hydrogen. Instead of shipping hydrogen directly, manufacturers will ship hydrogen-rich compounds such as ammonia and methane because they remain stable in more manageable environments. Once these compounds reach their destination, hydrogen can be extracted from the compound and used as fuel.
Electrochemical membrane reactors based on proton ceramic electrolytes offer distinct advantages for extracting hydrogen from hydrogen-rich compounds.
Traditional catalytic membrane reactors combine thermochemical catalysts, which facilitate decomposition of the compound, with mechanical pressure to drive hydrogen across a hydrogen-permeable membrane. The permeate emerges at a pressure lower than that of the feed, so additional mechanical pumps are needed to pressurize and compact the hydrogen for storage and transport.
In contrast, electrochemical reactors use voltage to drive hydrogen across a proton-conducting membrane. Because of the solid-state and gas-impermeable nature of the membrane, it is almost assured the hydrogen produced will be free of impurities. Plus, the hydrogen can be pressurized by just increasing the current, so additional equipment is not needed.
Despite recent advances in electrochemical membrane reactors, there are challenges to scaling up these devices for practical application, such as managing the temperature profile across the reactor.
“The process of pumping hydrogen across an electrochemical membrane leads to an increase in temperature because of the changes in hydrogen concentrations. At the same time, the decomposition reactions are inherently endothermic and drive the temperature down. Consequently, in a reactor with a simple linear flow, the upstream regime will be much cooler than the downstream regime. Such a temperature gradient introduces efficiency penalties,” Arthur J. Shih and Sossina M. Haile write in a recent perspective article.
Shih and Haile are postdoctoral scholar and professor, respectively, at Northwestern University. In their perspective article, they highlight recent work by researchers in Norway and Spain on a new reactor design that could improve thermal management.
The researchers come from CoorsTek Membrane Sciences, University of Oslo, and SINTEF Industry in Norway, as well as the Instituto de Tecnologia Química in Spain. This group is no stranger to electrochemical membrane reactor research. They previously developed a yttrium-doped BaZrO3 proton-conducting electrolyte for such reactors in 2017, which we reported on CTT.
In their recent study, they again used a BaZrO3-based electrolyte for the reactor, specifically yttrium-doped BaZrO3–BaCeO3. However, this time they also used multiphysics simulations and a new expansion-matched metal/glass-ceramic composite interconnect to create an optimized reactor architecture that allows internal heat exchange from exothermic to endothermic processes to minimize auxiliary heat input.
“[This] counterflow geometry … enables transfer of the heat generated at the downstream portion of the reactor, as a consequence of the electrochemical pumping, to the upstream portion of the reactor, where the carrier decomposition reactions cool the system,” Shih and Haile explain.
Plus, the new interconnect provides excellent heat transfer and electrical contact between adjacent cells in the reactor, and it matches the thermal expansion behavior of the components, contributing to the reactor’s long-term stability.
In addition, the researchers reported more than 99% hydrogen extraction efficacy for the system, which exceeds all other values in the literature. Energy loss is an inherent part of transforming energy from one form to another, so achieving such high extraction efficacy is impressive.
“The combination of hydrogen extraction efficacy and exhaust gas pressurization achieved in [Clark et al.’s] system is truly unprecedented,” Shih and Haile write. “Future efforts will likely be directed toward increasing the hydrogen flux, which remains moderate for their electrochemical system and does not factor into the extraction efficacy or pressurization metrics.”
In a CoorsTek press release, the researchers say a next step is to install a pilot plant hydrogen generator at Saudi Aramco’s headquarter campus in Dhahran, Saudi Arabia.
The paper, published in Science, is “Single-step hydrogen production from NH3, CH4, and biogas in stacked proton ceramic reactors” (DOI: 10.1126/science.abj3951).
The perspective, published in Science, is “Electrifying membranes to deliver hydrogen” (DOI: 10.1126/science.abo5369).
