[Image above] Credit: Rachel, Flickr (CC BY-NC 2.0)
I recently discussed with a glass manufacturer how phase diagrams are used in the glass industry. One particular piece of information surprised me—the compositional range of glasses designed for a specific application is often very narrow and complex, with many elements in the mix. Thus, measurement of the composition before, during, and after production are critical for ensuring processability and properties necessary for the end-use application.
Maintaining composition is particularly challenging with volatile components. The element fluorine provides functionality desirable for biomedical applications. Unfortunately, because reactions within glass melts can form gaseous silicon tetrafluoride (SiF4) or hydrogen fluoride (HF), it also is highly volatile. Measurement of fluorine can be made by labor-intensive titration or by energy dispersive X-ray spectroscopy, which has rather low sensitivity to light elements such as fluorine. Other methods available can require expensive equipment.
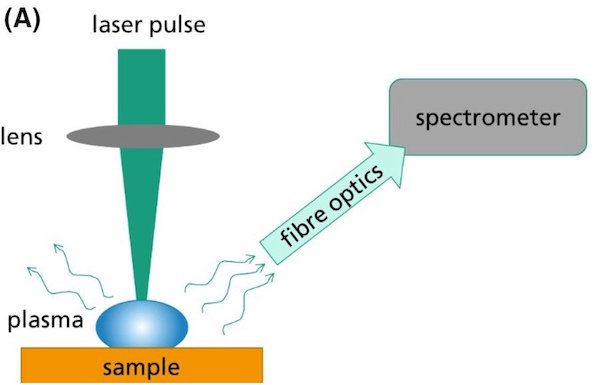
A recent open-access article appearing in the current issue of International Journal of Applied Glass Science (IJAGS) describes a new method for measuring fluorine in glass. Using short, high-intensity laser pulses focused on the surface of a sample, parts of the sample are heated, vaporized, and partly ionized at temperatures up to 10,000°C. Through spectroscopy, the radiation emitted by plasma provides information about the composition, with both qualitative and quantitative analysis possible with high sensitivity.
This measurement technique is relatively inexpensive and is minimally destructive. Furthermore, the measurements can be performed on both solids and liquids, making in-situ measurements feasible.
The authors used this method with calcium–silicate–phosphate glasses containing nominally fixed amounts of fluorine and calcium with varying ratios of phosphate and silicate. The spectroscopic data was calibrated against mixed powders of known compositions. The results indicated greater fluorine loss with higher concentrations of phosphates in the glass and compared favorably to literature studies on similar glasses.
The open-access paper, published in International Journal of Applied Glass Science, is “Fluorine loss determination in bioactive glasses by laser‐induced breakdown spectroscopy (LIBS)” (DOI: 10.1111/ijag.15867).
Another article in IJAGS, presently in Early View, explores a low-cost method for detecting bubbles in molten glass. Bubbles can have positive or negative effects on the properties of a glass, depending on the specific application. As with fluorine, current bubble measurement methods are labor intensive or require expensive equipment.
The authors of the Early View article used the ionic conductivity of the glass and the gas in the bubble to determine the relative fraction of bubbles in molten glass. Because the glass conducts and bubbles are insulating, it stands to reason that reduced conductivity can be attributed to the bubbles.
The conductivity of the glass is measured by dipping electrodes in molten glass. The conductivity of the bubble-free state is established by holding the glass at high temperature until conductivity reaches a maximum value plateau.
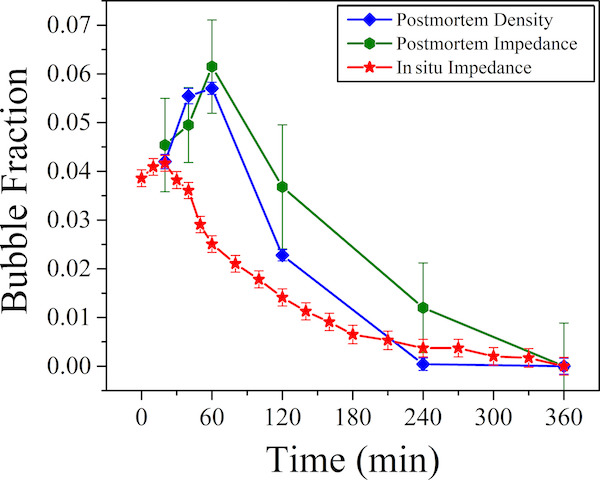
Results of the high-temperature impedance measurements are compared to post-mortem evaluations of bubble volume in the solid glasses using density and low-temperature impedance measurements.
The in-situ and post-mortem results were qualitatively similar, with maximum bubble fraction occurring at some time after the sample reached the set-point temperature. Unfortunately, the peak time and bubble fraction were different between in-situ and the post-mortem methods. Even with these differences, which the authors attribute to processes occurring during the quenching of the post-mortem samples, this study demonstrates the feasibility of high-temperature impedance spectroscopy. The benefits, including no sample preparation and real-time data, justify further work on this method.
The paper, published in International Journal of Applied Glass Science, is “Inferring bubble volume fraction in a glass melt through in situ impedance spectroscopy measurements” (DOI: 10.1111/ijag.15895).