[Image above] A self-sufficient glucose monitoring system developed by ETH Zurich researchers not only registers excess glucose in blood but initiates the release of insulin, thus helping to manage diabetes. Credit: Maity et al., ETH Zurich
Thanks to modern screening techniques and medicine, some chronic conditions are less fatal today than in the past, such as heart disease and stroke. Unfortunately, due to widespread lifestyle and societal changes, many chronic conditions are becoming more prevalent, such as diabetes.
Diabetes is a disease that impairs the body’s ability to produce or use the hormone insulin. This impairment results in abnormal metabolism of carbohydrates and elevated levels of glucose (blood sugar), which can damage blood vessels.
Lifestyle changes, such as managing your diet, stress levels, and sleep quality, can help regulate diabetes. However, in more severe cases, it is necessary to rely on oral medications and/or insulin injections to maintain proper glucose levels.
Insulin traditionally is administered through a syringe, injected directly into a layer of fat under the skin. But since the 1980s, people also have the option of using an insulin pump, a small device that automatically delivers small amounts of insulin throughout the day via a catheter, which needs changed every few days.
In either case, people need to manually check glucose levels to ensure the administered insulin is keeping blood sugar within the expected range.
Continuous glucose monitoring systems, which automatically track blood glucose levels, could pair with insulin pumps to automatically trigger the delivery of more insulin as needed. These devices may further alleviate the mental burden of diabetes management.
However, currently only a few continuous glucose monitoring systems are FDA approved. And like other bioelectronic devices, there are limitations to powering these systems.
“Currently available bioelectronic devices consume too much power to be continuously operated on rechargeable batteries, and are often powered wirelessly, with attendant issues regarding reliability, convenience, and mobility,” researchers write in a recent open-access paper. “Thus, the availability of a robust, self-sufficient, implantable electrical power generator that works under physiological conditions would be transformative for many applications.”
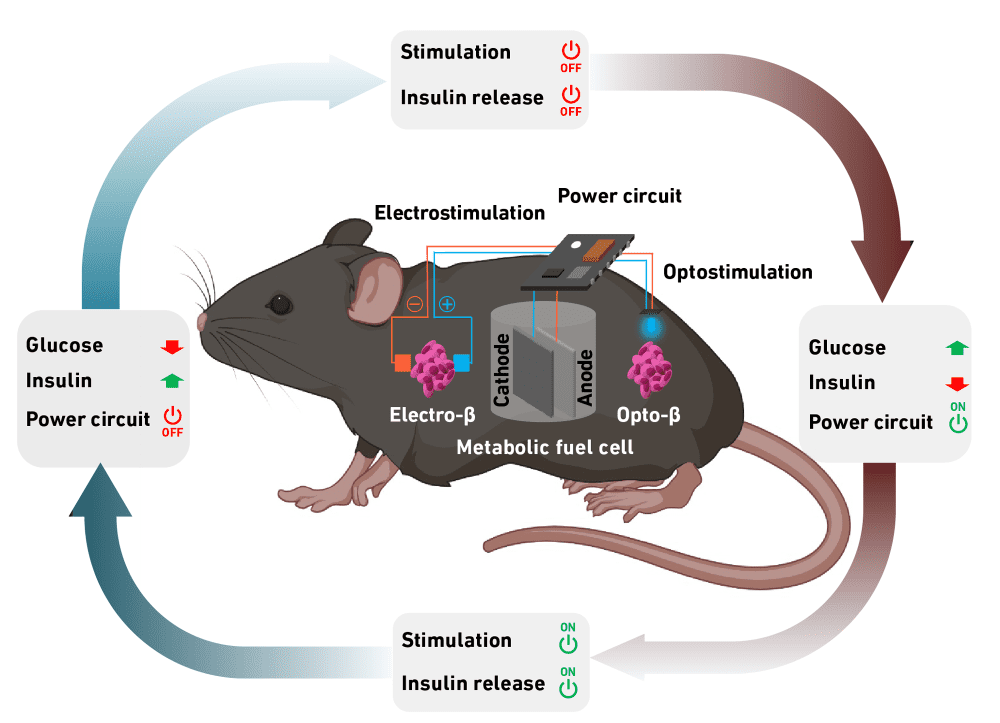
The researchers come from ETH Zurich in Switzerland. In their paper, they propose a self-sufficient glucose monitoring system that not only registers excess glucose but initiates the release of insulin into the blood.
The system consists of a fuel cell made from a copper-containing, conductively tuned 3D carbon nanotube composite. The fuel cell is encapsulated in alginate, an algae product approved for medical use, to insulate it from the tissue environment.
The alginate soaks up body fluid and allows glucose to pass from the tissue into the fuel cell. When the fuel cell registers excess glucose, it starts to generate electrical power. This energy is then fed to a capsule containing artificial beta cells, which produce and secrete insulin when stimulated with electricity or blue LED light. Once blood sugar falls below a certain threshold value, the production of electricity and insulin stops.
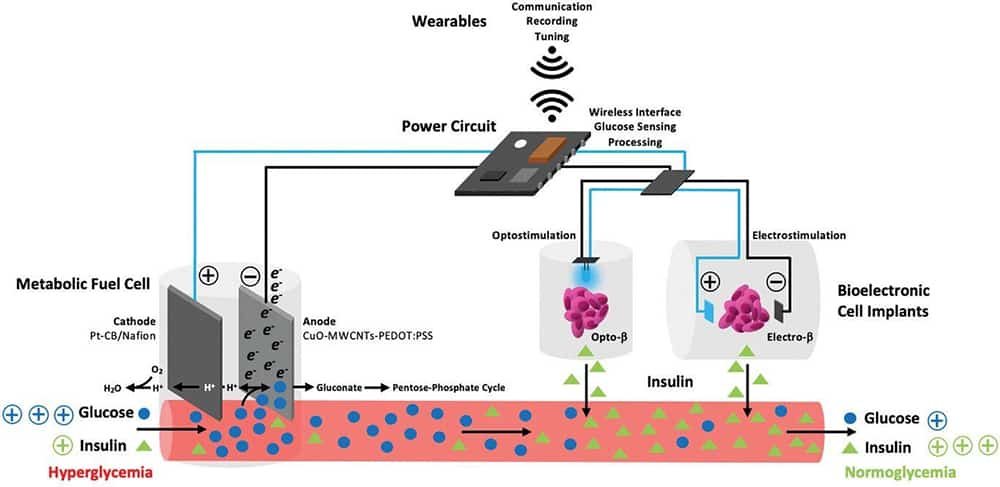
In addition to triggering insulin production, there is enough electrical energy provided by the fuel cell to enable communication between the implanted system and external devices, such as a smartphone. This connection would allow potential users to adjust the system via a corresponding app, as well as provide doctors with a way to remotely access and adjust the system.
The researchers validated their proposed system by testing the device in mice. However, “Bringing such a device to market is far beyond our financial and human resources,” says senior author Martin Fussenegger, professor of biotechnology and bioengineering at ETH Zurich. An ETH Zurich press release says further advancement of the technology would require an industry partner “with the appropriate resources and know-how.”
The open-access paper, published in Advanced Materials, is “Blood-glucose-powered metabolic fuel cell for self-sufficient bioelectronics” (DOI: 10.1002/adma.202300890).
