
[Image above] Could sucrose be an environmentally friendly alternative to phenolic resin binders in MgO-C refractories? Credit: David Goehring, Flickr (CC BY 2.0)
With the holiday season upon us, our kitchens will likely soon overflow with sugar, from frosted sugar cookies to turtle pudding cake to peppermint fudge. There are so many classic holiday sweets, my head just spins thinking of all the possibilities!
It is no surprise the food industry serves as the largest market for sucrose, the scientific name for common sugar. The medical industry (mainly pharmaceutical drugs) accounts for the second largest market, but after that, no other industry has a significant dependence on the compound.
But just because sucrose is used mainly as a food additive does not mean it cannot have other uses. At its core, sucrose is a binder, a fact that a team of Chinese researchers explored in quite a different industry—refractories.
MgO-C refractories
Magnesium oxide (MgO) is a widely used refractory material in furnaces and kilns due to its high melting point and strong corrosion resistance to alkaline slag. However, because of its large thermal expansion coefficient, MgO refractory products exhibit poor thermal shock stability.
To make MgO refractory products less susceptible to cracking, MgO is often combined with graphite to form MgO-C, a refractory material with improved porosity and thermal shock resistance.
Phenolic resin is a commonly used binder for MgO-C refractories. However, during the heat treatment process, phenolic resin releases pollutant gases, such as methyl aldehyde and phenols, which have harmful effects on both health and the environment.
Replacing phenolic resin with an environmentally friendly binder would be ideal—and sucrose may just be that binder.
Sucrose as binder for MgO-C refractories
In an open-access paper published in IOP Conference Series: Materials Science and Engineering, researchers from China University of Geosciences and Hunan University of Humanities, Science and Technology in China used sucrose solution instead of phenolic resin as binder in MgO-C refractories.
They created the refractories using fused magnesia and flake graphite as the main raw materials and added metal aluminum powder as an antioxidant. They prepared refractories with both phenolic resin and sucrose as binders to compare.
The researchers first studied the carbonization behavior of the sucrose binder using infrared spectroscopy and differential thermogravimetry.
“[I]t is known that free water and some easily decomposable substances in sucrose are decomposed in the temperature range from room temperature to 320°C; sucrose decomposes into CO2 and H2O in the temperature range from 320 to 800°C, and thermogravimetric curves are basically stable in the temperature range from 800 to 1400°C, indicating that sucrose has been decomposed, and carbonization completely produces a stable carbon structure in this temperature range,” they write.
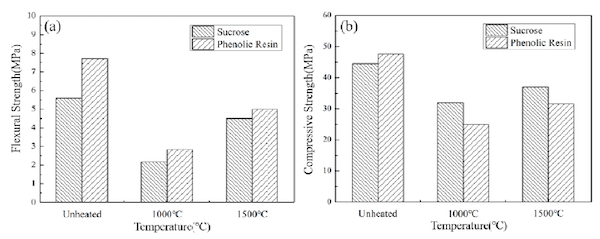
Knowing this information helped them understand flexural strength and compressive strength measurements. Specifically, sucrose-bonded MgO-C refractories showed higher flexural (5.59 MPa) and compressive (44.50 MPa) strengths when heat-treated at 180°C, lower strengths when heat-treated at 1,000°C, and increased flexural (4.51 MPa) and compressive (37.00 MPa) strengths when heat-treated at 1,500°C.
The researchers then compared flexural and compressive strengths of sucrose-bonded MgO-C refractories to refractories bonded with phenolic resin.
“Comparing the flexural strength and compressive strength of MgO-C refractories prepared with phenolic resin as binder, it can be seen that the flexural strength and compressive strength of the specimens obtained with phenolic resin as binder are slightly higher than those obtained with sucrose as binder after curing at lower temperature,” the researchers write, “but overall, the difference was not large.”
“Therefore, it is feasible to substitute phenolic resin with low-cost and environmentally friendly sucrose solution as binder for MgO-C refractories,” they conclude.
Who knew the compound that makes your desserts taste so good could also offer a sweet deal to the refractories industry? I’ll definitely view my sugar cookies differently from now on!
The open-access paper, published in IOP Conference Series: Materials Science and Engineering, is “Preparation and properties of MgO-C refractories using sucrose as binder” (DOI: 10.1088/1757-899X/678/1/012092).
