
[Image above] Diamond is the hardest machining material currently available, but researchers are working to develop synthetic materials that can match or surpass this limit. Credit: Emilian Robert Vicol, Pixabay
By Laurel Sheppard
In the recent April 2024 Bulletin, numerous articles explored the ceramics used for machining and manufacturing other materials and components. And as noted in the “Business and Market View” column on abrasives, nothing beats diamond when it comes to grinding effectiveness due to its exceptional hardness.
Even though diamond stands supreme among naturally formed materials in terms of hardness, scientists have continually aimed to overcome this natural limit through synthetic fabrication of different carbon- and nitrogen-based materials. Wurtzite boron nitride (wBN) is the most advanced example of this goal. First synthesized in 1963, wBN can now be stabilized at atmospheric pressure to obtain high-quality samples, though these efforts are still a long ways from scaleup and commercialization.
Since 1989, carbon nitrides have captured the attention of many research groups due to theoretically predicted potential for extreme hardness. Specifically, carbon nitrides featuring three-dimensional frameworks of CN4 tetrahedra are expected to have a hardness greater than or comparable to diamond.
Yet over the past three decades, attempts to synthesize these carbon nitrides using a variety of methods resulted in only a single success in 2016. In that study, researchers obtained a fully saturated sp3-hybridized carbon nitride (the CN compound) in a diamond anvil cell laser-heated to 7,000 K at pressures more than 55 GPa. However, this compound was unstable below pressures of 15 GPa and could not be recovered at ambient conditions.
Now, in a recent groundbreaking study, researchers from numerous universities across Europe reported that they successfully synthesized four covalent carbon nitrides and could recover them all at ambient conditions.
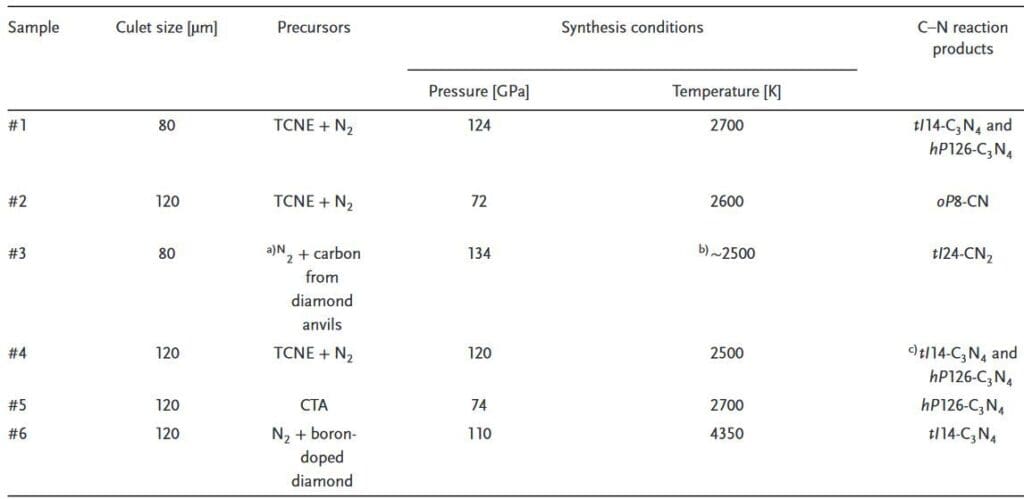
The four covalent carbon nitrides reported in the study include the previously synthesized CN composition (oP8-CN), two with the C3N4 composition (tI14-C3N4, hP126-C3N4) and one with the CN2 composition (tI24-CN2). All the compounds feature 3D polymeric structures in which C and N atoms are fourfold and threefold-coordinated, respectively.
The authors synthesized the samples using different precursors at pressures between 70 to 140 GPa and temperatures above 2,000 K, as detailed in the table below. In all cases, synthesis of the carbon nitrides was performed in laser-heated diamond anvil cells at their target pressure.
Chemical reactions occurred in all sample conditions, resulting in formation of the four carbon nitrides. The average N–C–N (or C–C–N) bond angle was 109.5°, which matches the value expected of an ideal tetrahedron.
A variety of analysis methods were used to determine the materials’ structures and properties, including synchrotron single-crystal X-ray diffraction (SC-XRD), Raman spectroscopy, scanning electron microscopy, and transmission electron microscopy. Density functional theory calculations, which predict material behavior based on quantum mechanics, were also used to confirm the high bulk modulus of the materials.
These analyses revealed that all four compounds have hardness values comparable to either cubic boron nitride or diamond, depending on the employed hardness model. Besides their superhardness, these strongly covalently bonded materials also exhibit
- High thermal conductivity and energy density,
- Wide bandgaps and insulating properties,
- Second-order harmonic generation, and
- Possible tunability of photoluminescence via defects.
Additionally, the compounds tI14-C3N4 and tI24-CN2 can exhibit piezoelectricity due to their non-centrosymmetric structures. The combination of piezoelectricity and superhardness of these two carbon nitrides makes them attractive for smart and resilient cutting tools.
After opening the diamond anvil cells and exposing the samples to air, high-quality SC-XRD data could still be collected at ambient conditions, which means the nitrides’ crystallinity and high-pressure crystal structures were preserved. According to the authors, this is the first time such complex materials synthesized above 100 GPa were recovered.
In a University of Edinburgh press release, first author Dominique Laniel, a Future Leaders Fellow at the University of Edinburgh, says the researchers were at first “incredulous” to have produced materials that researchers were dreaming of for the last three decades. But now with their existence confirmed, “These materials provide strong incentive to bridge the gap between high pressure materials synthesis and industrial applications,” he says.
Coauthor Florian Trybel, assistant professor at the University of Linköping, elaborates further on the study’s novelty in the university press release, stating“These materials are not only outstanding in their multifunctionality but show that technologically relevant phases can be recovered from a synthesis pressure equivalent to the conditions found thousands of kilometers in the Earth’s interior. We strongly believe this collaborative research will open up new possibilities for the field.”
The authors write that future directions for research include investigating alternative synthesis methods for these compounds. For example, it may be possible to use small amounts of crystals pre-synthesized as seeds to grow the carbon nitrides in mild conditions in large-volume presses or by chemical vapor deposition.
The authors conclude the study by noting that this discovery of C3N4 compounds with fourfold-coordinated carbon atoms “could also serve as a key starting point for the search for a solid featuring the thus far elusive six-fold coordinated carbon atom, analogous to silicon in the high-pressure compound 𝛾-Si3N4.”
The open-access paper, published in Advanced Materials, is “Synthesis of ultra-incompressible and recoverable carbon nitrides featuring CN4 tetrahedra” (DOI: https://doi.org/10.1002/adma.202308030).
