[Image above] A droplet of water beads up on top of a hydrophobic surface. Credit: BYU
Ride of the water droplets. Credit: National Science Foundation; YouTube
“Surface properties are key for any sensitive application, because the surface controls the interaction with the outside world,” materials scientist Art Baddorf says.
Baddorf, a scientist at Oak Ridge National Lab’s Center for Nanophase Materials Sciences, knows a thing or two about surfaces. (More on that later.)
Like he said, materials’ surfaces are really important because they influence how something interacts with the rest of the world.
Much attention of late has been given to how surfaces interact with liquids, particularly water—whether they cozy up to (hydrophilic) or shy away from (hydrophobic) those water molecules. Watch the video above to see some footage of some of these rather impressive surfaces.
We’ve written before about hydrophobic surfaces that promise to keep your car, camera, or solar cells clean, and even hydrophilic surfaces that attempt to keep glass clear.
Many of these so-called “self cleaning” surfaces have a coating them keeps they clean by preventing water molecules from lingering on the surface. Several companies have commercialized special coatings for a range of products and applications, including Hydrobead, NeverWet, Ultra-Ever Dry, and LiquiGlide (the “permanently wet, liquid-impregnated surface” that keeps ketchup moving, among other things).
But another way to manipulate surface interactions is to fabricate miniscule structures on the materials’ surface.
Inspired by nature (lotus leaves and Namibian desert beetles, for example), surface nanostructures that convey these properties have come a long way from their humble beginnings. “Ten years ago no one had the ability to pattern surfaces like this at the nanoscale,” Sumanta Acharya, a National Science Foundation (NSF) program director in the Division of Chemical, Bioengineering, Environmental, and Transport Systems, says in an NSF Discoveries report.
But in just a decade, a lot has changed.
Superhydrophobic surfaces revealed
Scientists now have both the design knowledge and fabrication ability to synthetically pattern nanoscale structures onto materials’ surfaces.
Such improvements have been made that the newest surfaces aren’t just hydrophilic or hydrophobic—they’re superhydrophilic and superhydrophobic.
Brigham Young University mechanical engineering professor Julie Crockett researches superhydrophobic surfaces. Her team of scientists has found that combining the best of both water-fearing worlds—nanostructures and surface coatings—can significantly boost water resistance.
Crockett’s team is testing both surfaces with little posts (which look like microscopic peg boards) and surfaces with ribs and cavities to see how each structure influences interaction with water droplets.
Their research shows that altering the width of these structures can have a big impact on its interaction with water. And understanding these interactions is the key to designing new materials with improved functions and performance.
“People know about these surfaces, but why they cause droplets or jets to behave the way they do is not particularly well known,” Crockett says in a BYU press release. “If you don’t know why the phenomena are occurring, it may or may not actually be beneficial to you.”
Watch the video below to hear more from Crockett about the work.
Credit: BYU; YouTube
Insights into oxide surface structures
Understanding surface structures goes beyond explaining and designing how surfaces interact with water molecules, though. As ORNL scientist Baddorf said earlier, the surfaces are important because they influence how a material interacts with the world.
Baddorf and a team of ORNL scientists recently published a paper in Nature Communications detailing the surface structure of oxide materials.
Using high-resolution scanning tunneling microscopy to scrutinize the surface of perovskite manganite, a material that exhibits—“dramatic magnetic and electronic behavior”—the scientists showed that a surface’s chemical and physical properties are heavily intertwined.
“It’s like the material has many knobs, and if you turn one, all the properties change,” lead author Zheng Gai says in a press release. “You turn a different knob and the whole thing changes again. It turns out the surface is another knob—you can use it to change the properties.”
The team created distortion maps that showed the position of oxygen atoms on the material’s surface, revealing that they are arranged into a checkerboard pattern or Jahn-Teller distortion.
“The oxygen totally changes the surface energy,” Gai says. “Once you introduce oxygen, the electrons don’t like to form a straight line; they zigzag to get to a lower energy state. This distortion is a very common concept in bulk materials, but nobody has been able to show this effect on the surface before.”
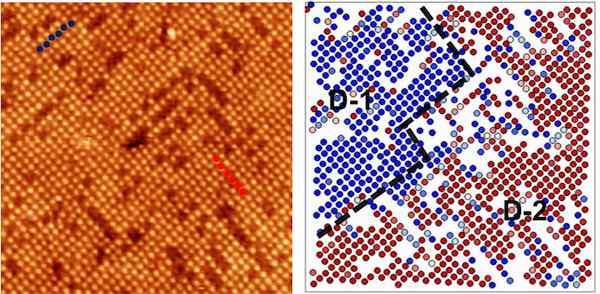
A distortion map (right) shows structural domains that were not easily identified in raw images (left) of an oxide surface. Credit: ORNL
Applications for superior surfaces
Understanding and manipulating a material’s surface properties are critical for applications from cell phone screens that resist smudging to boots that keep clean. But beyond an endless array of consumer products, there’s also a lot of room for practical advances with potentially big impacts.
One big business application of superhydrophobic surfaces is for more efficient condensers. Condensers—present in energy-intensive devices like automobiles, appliances, and even power plants—depend on condensation of water droplets on their surfaces. But that very process of developing a film of water on the surface dramatically reduces the ability of more water molecules to condense on the surface.
Giving a condenser a superhydrophobic surface, however, would allow condensed water droplets to wick away as they form, boosting the efficiency of the condenser to save considerable energy and extend the device’s life.
Companies like NBD Nanotechnologies are working on scaling surface coatings, so we may soon see these surfaces incorporated into industrial settings like power plant condensers.
Superior surfaces also have applications in materials that resist icing, like aircraft wings and wind turbines (although some report that the effectiveness of these advances are questionable), and simple medical diagnostic devices.
And superhydrophobic ceramics for a range of purposes? You bet.
Wondering how to use nanostructures to change your haircolor? (It’s ok, you can tell us!)
That’s also possible, according to a report from the University of New Mexico.
Using an ion beam, scientists patterned human hairs with diffraction gratings that reflect light at particular wavelengths, making hair appear a specific color. Sure there are more practical applications, too—like security markings for identification of people or property—but what fun is that?