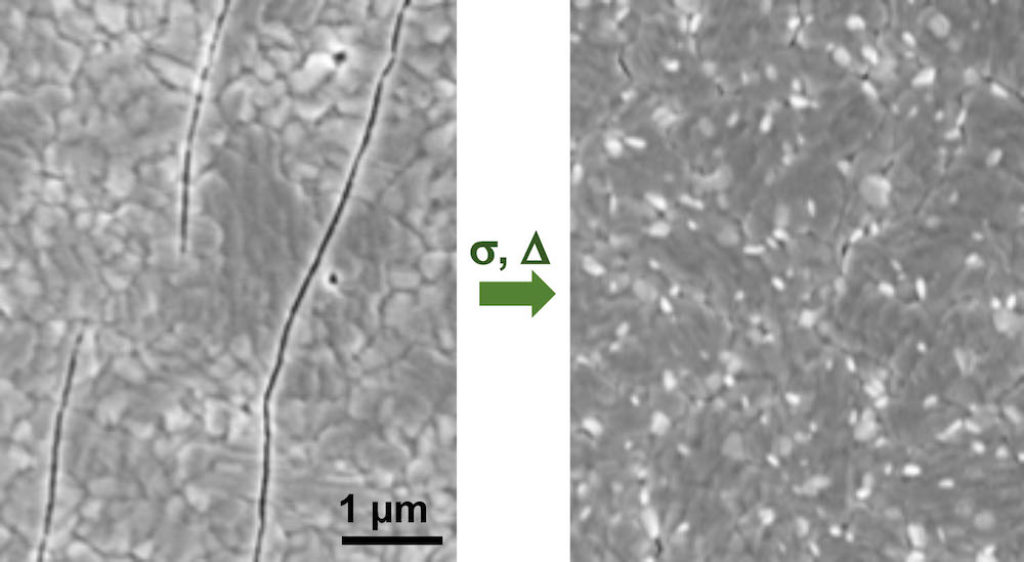
[Image above] With just some compression (σ) or a little heat, a cracked perovskite film (left) can be fully healed (right). Credit: Padture Lab, Brown University
It’s been a while since we last talked about perovskites.
The term “perovskite” traditionally refers to a specific mineral, calcium titanium oxide. But the term also currently applies to the class of halide compounds that have the same type of crystal structure as CaTiO3, known as the perovskite structure. The latter definition is what scientists refer to when talking about perovskite solar cells (PSCs).
PSCs are expected to become a leading contender to silicon-based solar cells, which currently account for over 90% of the solar cell market. They will be used either by themselves or in tandem with silicon-based solar cells.
When comparing PSCs and silicon-based solar cells in terms of efficiency, cost, and versatility, PSCs offer advantages.
- Efficiency—PSC efficiency rose from about 4% in 2009 to just over 25% in 2019, which puts their efficiency (for lab-scale cells) almost on par with single-crystal silicon solar cells. Scientists expect that efficiency to continue increasing. (Some tandem perovskite-silicon solar cells already outdo traditional silicon.)
- Cost—Compared to traditional silicon-based panels, which cost about 50 cents per watt, some estimates put PSC cost at just 10–20 cents per watt. This difference is due to the fact that perovskites are processed at relatively low temperatures compared to silicon cells, where single crystals of silicon need to be grown from a melt at high temperatures to remove defects (a costly process).
- Versatility—Unlike silicon wafers, which tend to be thick, heavy, and rigid, perovskite films have a thin, flexible, and lightweight structure, so they are more easily incorporated into a variety of places, including roof shingles, windows, tents, and other surfaces.
Yet there are challenges to commercializing PSCs, including addressing potential environmental impacts of PSCs containing lead. But one challenge in particular has gained frequent attention of late—durability.
“In materials science, things that are easy to make also tend to be easy to break,” says Nitin Padture, the Otis E. Randall University Professor and Director of the Institute for Molecular and Nanoscale Innovation at Brown University, in a Brown press release. “That’s certainly true of perovskites, which are quite brittle.”
Padture, an ACerS member since 1985 and Fellow since 2005, began studying organic-inorganic halide perovskites (OIHPs) when he moved to Brown University in 2012. Before then, he primarily studied structural ceramics and some electronic nanoceramics. “I’d always had my eye on that field [photovoltaics], but never quite had the chance to get into it,” he says in a phone call. But the move to Brown gave him the opportunity.
Padture also wanted to study the mechanical behavior of PSCs, something that is largely overlooked in the literature but is vitally important to PSC reliability. And with his background in fracture mechanics, Padture had a hunch of how to address perovskite brittleness.
“I knew that things that are easy to make are also easy to break because the formation energy of materials is low,” he says. But “that means you can also fix them easily!” he adds.
In a recent study Padture conducted with doctoral students Srinivas Yadavalli and Zhenghong Dai, assistant professor (research) of engineering Yuanyuan Zhou, and Argonne National Laboratory physicist Hua Zhou, they looked to heal cracks in OIHPs using two simple techniques—moderate compression or heat.
They used two typical OIHPs for the study: methylammonium lead triiodide (CH3NH3PbI3 or MAPbI3) and formamidinium lead triiodide (α-HC(NH2)2PbI3 or α-FAPbI3).
The MAPbI3 thin films were synthesized using a solvent-engineering method while the α-FAPbI3 thin films were synthesized using the Lewis acid-based adduct approach. Both were deposited on polyethylene terephthalate (PET) plastic substrates.
To test the viability of mechanically healing perovskite thin films, the researchers wrapped the thin films on the PET substrates around a glass mandrel. “This procedure, rather than bending the substrate without the mandrel, ensures constant bending radius over the entire specimen and, thus, a uniform applied uniaxial stress,” they write.
The applied stress was either tensile or compressive depending on the curvature—tensile when films faced away from the mandrel (causing the film to crack), and compressive when films faced toward the mandrel (causing, hopefully, the film to heal).
The researchers also tested the viability of thermally healing different samples of perovskites by heat-treating MAPbI3 and α-FAPbI3 thin films at 100°C for 5 minutes and 140°C for 10 minutes, respectively.
Using X-ray diffraction, the researchers confirmed that compressive stress at room temperature or heat treatment at moderate temperatures healed cracks in the thin films on a time-dependent basis.
“In the case of mechanical-healing, an externally applied compressive stress σc = –214 MPa is sufficient to bring the crack walls in intimate contact in both MAPbI3 and α-FAPbI3 thin films. Restoration of chemical bonding can then occur as a result of stress-induced, thermally-activated ionic-diffusion across the closed interface,” they write.
“In the case of thermal-healing, where no external compression is applied, … internal compressive stresses induced due to the heat-treatment are lower than the applied stress of σc = –214 MPa in the room-temperature mechanical-healing case, but the higher temperature is expected to accelerate ion-diffusion, resulting in the crack healing in the OIHP thin films,” they add.
Padture says this proof-of-concept study lays the groundwork to move beyond thin films and investigate healing PSCs with these techniques. In addition, Padture says what they learned about cracks in this study will help them investigate fracture at the interface between the films and substrate.
The vertical cracks within the films provide “sort of a free surface for the horizontal interfacial cracks to form,” Padture says. So even though film cracks may not really affect cell efficiency on their own, “they may have a catalytic effect of promoting interfacial cracks, which are more dangerous.”
The paper, published in Acta Materialia, is “Facile healing of cracks in organic–inorganic halide perovskite thin films” (DOI: 10.1016/j.actamat.2020.01.040).
