
[Image above] Graduate researcher assistant Xiaofei Zhao prepares a Ti3C2 MXene dispersion in sodium L-ascorbate solution. Credit: Dharmesh Patel, Texas A&M
If you are a regular reader of CTT, you probably recognize the term MXene from multiple articles we published last year.
MXenes are a family of 2D transition metal carbides, carbonitrides, and nitrides that are made by selectively etching MAX phases. Like graphene, MXenes have promise in a wide range of applications, including energy storage and water purification, and EMI shielding and sensing.
However, one property of MXenes presents a challenge to bringing MXene-based technologies to fruition—MXenes rapidly degrade when kept in the open.
Several past studies have looked at factors that drive MXene oxidation. For example, oxidation rates are substantially higher in aqueous phases compared with organic solvents, air, and solid media.
Another factor influencing oxidation is sample thickness—individual nanosheets oxidize more easily than multilayered MXene clay. Yet individual nanosheets typically show better electrical performance, colloidal stability, and processability than multilayered MXenes.
So, new techniques are sorely needed to increase oxidation stability of individual nanosheets.
Current strategies for mitigating MXene oxidation have mostly involved restricting oxygen exposure or freeze-drying MXenes under vacuum. However, Texas A&M professor of materials science and engineering Miladin Radovic explains these techniques are not ideal.
“Restricting oxygen exposure is not an option for most of the potential applications of MXenes,” he says in an email.
In a recent paper published in Matter, researchers from Texas A&M led by Radovic and associate professors of chemical engineering Micah Green and Jodie Lutkenhaus propose a new technique, one that could extend MXene shelf life by years.
In their study, the researchers exposed MXenes to antioxidants. In an email, Green says industry partners had been urging the researchers to find some solution that would give MXenes long shelf stability, and they decided to test antioxidants because “[t]hese antioxidants have proven effective in other applications.”
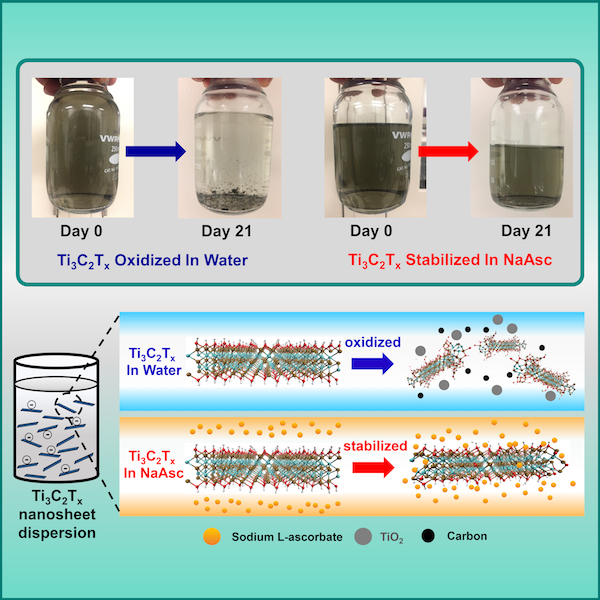
The researchers focused on Ti3C2Tx, the most common MXene. They diluted colloidal Ti3C2Tx nanosheet dispersions with a premixed aqueous solution of sodium L-ascorbate (NaAsc) and then stored the NaAsc-stabilized dispersions for 21 days in closed bottles at ambient temperature.
X-ray diffraction patterns of NaAsc-stabilized dispersions and control dispersions made with deionized water showed marked differences. “[T]he colloidal Ti3C2Tx nanosheets retained their pronounced (002) peak at a 2θ angle of around 6.5°,” the researchers write in the paper. “However, the (002) peak disappeared completely from the XRD spectrum for Ti3C2Tx stored in deionized water, indicating the total oxidation of crystalline MXene to an amorphous structure.”
“Collectively, these XRD results suggest that the crystalline structure of Ti3C2Tx nanosheets can be effectively retained by introducing an antioxidant,” they state.
The researchers conducted several other tests, such as measuring changes in electrical conductivity and chemical composition, to complement their X-ray diffraction findings. Taken together, the tests supported the conclusion that antioxidants restrict oxidation in MXenes.
Why do antioxidants so effectively restrict oxidation? According to the researchers, “The origin of this stability against oxidation is attributed to the association of L-ascorbate anions to the edges of Ti3C2Tx nanosheets, which prevent otherwise detrimental oxidation reactions.”
NaAsc is not the only antioxidant that associates with reactive sites on the MXene nanosheets. The researchers tested several related compounds, including vitamin C, and found these antioxidants offer the same protection.
In a Texas A&M press release on the research, Green notes the researchers made the discovery about a year ago, and the treated MXenes are still stable.
Radovic says they are currently testing the effectiveness of this method for other MXenes and also testing some other antioxidants. “[F]irst results are quite promising,” he says.
The paper, published in Matter, is “Antioxidants unlock shelf-stable Ti3C2Tx (MXene) nanosheet dispersions” (DOI: 10.1016/j.matt.2019.05.020).
Update 07/23/2019 – Article updated with additional information received from Radovic and Green
