[Image above] Graduate student Chih-Chau Hwang, left, and Professor James Tour show a vial of material that has the ability to capture carbon dioxide from gas flowing out of a well and hold it until it can be otherwise used. Credit: Jeff Fitlow; Rice University
Natural gas, in comparison to its cousins coal and oil, is a relatively clean fossil fuel. However, its recovery and preparation pose several challenges that start to drag on its cost, energy, and environmental impact.
Ceramic proppants can offer solutions to some of the challenges with gas recovery, as written about in the January/February issue of the ACerS Bulletin, although there are additional difficulties in collection and use of the gas once out of the well.
A new material developed by scientists at Rice University may help ease some of those burdens by replacing current costly and energy-intensive techniques to isolate natural gas from contaminating gases that also are extracted from wells. The advantages of their new technique is that the material is easy and economical to produce, and the actual separation doesn’t require a large energy input, improving many of the current downsides to natural gas production.
“Most people don’t realize that natural gas, when it comes out of the ground, is contaminated 10–20% by CO2,” chemistry professor James Tour says in a Rice video (below). “Sometimes, in some parts of the world, as much as 70% of natural gas coming out of the ground is CO2.”
Credit: Rice University; YouTube
Current methods to remove CO2 are either energy-intensive (e.g., cryogenic separation) or require expensive materials (e.g., zeolite filters). One frequently used method involves bubbling natural gas through large towers of corrosive liquid amine solvent. The liquid must to be heated to high temperatures (140°C) to remove the CO2, which is then often vented to the air in lieu of better sequestration methods. These current common practices therefore require a lot of space and energy and further contribute to accumulation of greenhouse gases in the Earth’s atmosphere.
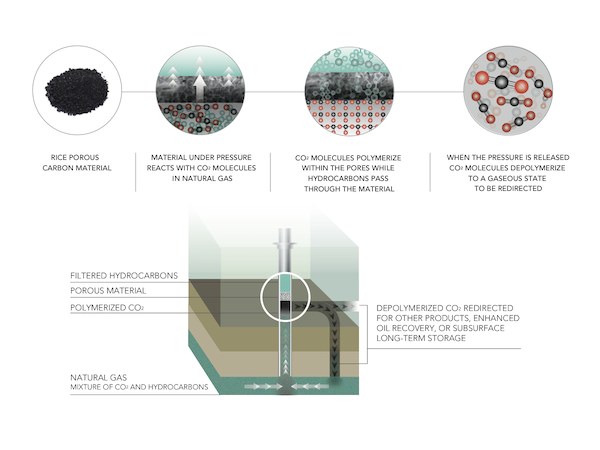
The new research, published in Nature Communications, details the development of a nanoporous carbon-nitrogen or carbon-sulfur solid that can strip CO2 from natural gas at ambient temperatures. The process polymerizes the CO2 molecules, providing very efficient capture of the greenhouse gas. Tour says the growing polymer chains of carbon dioxide prevent methane, ethane, and propane molecules in the natural gas from also sticking to their material.
“This will enable companies to pump carbon dioxide directly back downhole, where it’s been for millions of years, or use it for enhanced oil recovery to further the release of oil and natural gas. Or they can package and sell it for other industrial applications,” Tour says in a university press release.
To create the wonder materials, the researchers treated carbon with potassium hydroxide at 600°C, forming powders of porous material containing carbon-nitrogen or carbon-sulfur atom mixtures. The carbon-sulfur product absorbed 82 percent of its weight in CO2, and the carbon-nitrogen product performed nearly as well, according to the press release.
The carbon material removes CO2 under pressure from the gas flow, but spontaneously depolymerizes when the pressure is released, allowing efficient reuse over and over again. Tour says in the release that the material is very stable, showing no degradation after many cycles. With a surface area of 2,500 m2/g, it’s “enormously robust and extremely stable,” he says.
“Our technique allows one to specifically remove carbon dioxide at the source. It doesn’t have to be transported to a collection station to do the separation,” Tour says. “This will be especially effective offshore, where the footprint of traditional methods that involve scrubbing towers or membranes are too cumbersome.”
Want to hear more about the science behind the process? Listen to Tour go into the molecular nitty gritty in the video below.
The research was done in collaboration with the National Institute of Standards and Technology and was funded by oil and gas exploration and production company Apache Corp.
The paper is “Capturing carbon dioxide as a polymer from natural gas” (DOI: 10.1038/ncomms4961).
Credit: Rice University; YouTube