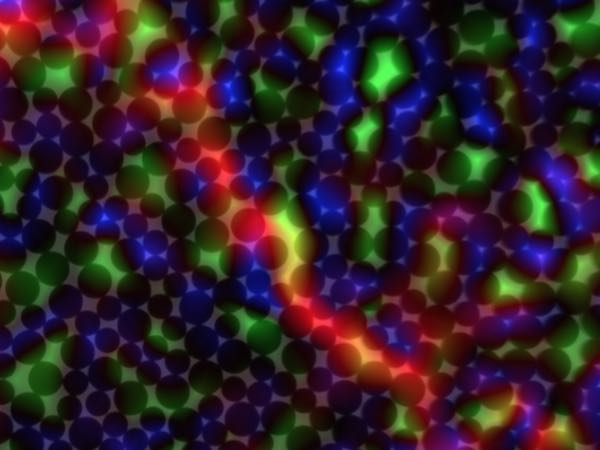
[Image above] An image of a 2-D granular system that University of Pennsylvania researchers studied to better understand failure in disordered solids. Blue shows overpacked regions, green shows under packed regions, and red shows a transient shear band. Credit: University of Pennsylvania
Glass can break spectacularly.
And when it breaks, its molecules—which are arranged in a somewhat disordered, amorphous state—rearrange a bit. But how do we tell which ones will rearrange, and how?
In crystalline solids, the story is much more straightforward—defects within the regular crystal structure inform precisely where these changes will occur.
“In order to understand how a system chooses its rearrangement scenario, we must make connection with the underlying microscopic structure,” Douglas Durian, professor of physics and astronomy at the University of Pennsylvania, says in a university press release. “For crystals, it’s easy; rearrangements are at topological defects such as dislocations. For disordered solids, it’s a very hard 40-year-old problem that we’re now cracking: What and where are structural defects in something that’s disordered?”
Durian and an interdisciplinary group of scientists at the University of Pennsylvania have harnessed intense computation, data, and modeling power to determine how disordered solids fail, an understanding that could help engineer custom materials, such as glass that is less likely to break.
Using machine learning, the scientists analyzed patterns of how atoms rearrange in a slew of different materials and systems spanning several disparate length scales. Part of the power of the analysis lies in its incorporation of a wide range of material systems—spanning size scales of individual atoms to river rocks—and a combination of data from 15 simulations and experiments on various disordered solids.
The team analyzed extensive data on how the particles are arranged in each system and how those particles respond to stresses, such as thermal alteration or squeezing. That mountain of data allowed the scientists to predict how likely individual particles are to rearrange with the system, a microscopic structural property called softness.
By measuring spatial correlations and strain response of softness, in addition to rearrangement size and yield strain, which are both measure of plasticity, the scientists found that these quantities were remarkably similar—even across wide-ranging disordered solids spanning 7 orders of magnitude in diameter and 13 orders of magnitude in elastic modulus. In other words, very different disordered solid systems nonetheless behave very similarly.
“People have been talking about what sets the size of localized rearrangements in disordered solids for 40 years,” Andrea Liu, Hepburn Professor of Physics at the University of Pennsylvania, says in the release. “They speculated about localized defects that they called shear transformation zones in disordered systems where rearrangements are likely to occur, but no one had seen this directly. They couldn’t predict ahead of time where rearrangements would be likely to occur. With the machine learning, we’re saying, ‘Let’s train the system. Let’s look at the rearrangements and the structures and see if we can figure out what’s important and then use that.’ It’s conceptually very straightforward, but it turns out to be very powerful.”
Although that powerful analysis revealed that various disordered solids are rather similar in terms of how rearrangements occur, how the material then responds to those rearrangements dictates the very different responses materials can have to these stresses.
“When a rearrangement happens, the softnesses of the nearby particles all change, but, due to long-range elastic couplings, so can the softnesses of particles even quite far away, as illustrated by this data,” Durian says in the release. “Thus, a rearrangement has a nontrivial effect on where the next rearrangements are likely to occur. In particular, will nearby rearrangements be encouraged and hence promote shear banding, or will they be discouraged and hence promote toughness? We believe that understanding and ultimately controlling the complex interplay between rearrangements, stress, and structure—here quantified by softness—is the key to improving toughness.”
And that’s a common materials science goal, as improving toughness can help engineers design materials that are less likely to to fail. When it comes to glass, that means smartphone screens that are less likely to shatter, windows that need replaced less often, and perhaps even new applications for a what is viewed as an otherwise traditionally brittle material.
“Disordered solids have a lot of great properties,” Liu says in the release. “You can mold them into any shape you want or create surfaces that are atomically smooth, which you can’t really do with crystalline systems. But they tend to shatter easily. If we can understand what controls that and how to prevent it, then the concepts start to have real applications. In an ideal case, we want to develop new, tougher materials that aren’t as brittle or don’t fall apart as catastrophically.
The paper, published in Science, is “Structure-property relationships from universal signatures of plasticity in disordered solids” (DOI: 10.1126/science.aai8830).
Did you find this article interesting? Subscribe to the Ceramic Tech Today newsletter to continue to read more articles about the latest news in the ceramic and glass industry! Visit this link to get started.
