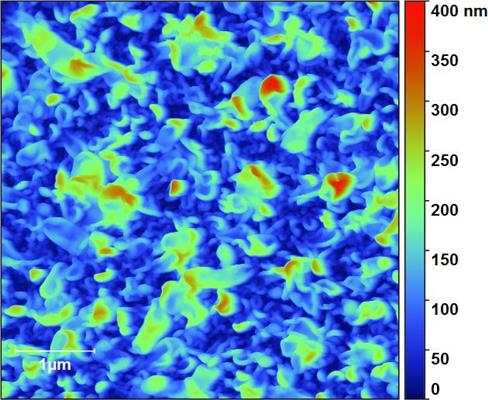
[Image above] ETH researchers engineered free-standing ceramic membranes for so-called micro energy converters. The strain patterns of these membranes control their properties. Credit: Shi Y et al. Nature Materials 2015
Scientists at ETH Zurich have found that doping is not the only way to influence ion conductivity in ceramic membranes.
Doping, or adding impurities to a material to influence its charge-carrying capacity, is traditionally exploited to customize the conductivity of membranes, but it has its limits. And adding an impurity can alter the membrane’s properties, too—sometimes that’s a good thing, and sometimes it’s not.
Instead, the Zurich team showed that it could control ion conductivity by generating strain in a thin film of gadolinium-doped ceria (Ce0.8Gd0.2O1.9–x) to create wrinkles.
Thin ceramic membranes are integral components in solid oxide fuel cells, solid state batteries, industrial filters, and numerous other technologies, so the potential implications of better and more versatile membranes are far-reaching.
“For the first time, scientists can now selectively manipulate the buckling profile, and thus the physical properties, allowing new technical applications of these membranes,” according to an ETH Zurich press release.
Although previous research has shown that strain can alter ion transport, the Zurich team is first to definitely show this effect in free-standing films (rather than those supported on a substrate) and first to effectively control conductivity by altering strain.
The team fabricated ceramic films sans substrate “by free-etching the substrate under the thin ceramic layer,” according to the release. This process created a contorted membrane, which the team hooked up to platinum electrodes.
Measuring the conductivity of these free films showed that the electrode pattern controls the film’s net strain, “which is of interest for device engineering to actively manipulate the electro–chemo–mechanics beyond classic doping strategies,” the paper states.

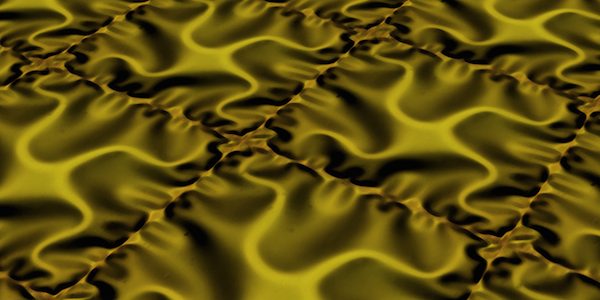
Microscopic photos show how the buckling profile of ceramic membranes depends on how the platinum microelectrodes are attached to the membranes. Credit: Shi Y et al. Nature Materials 2015
In addition to guiding engineering of future membranes, the work can also help complete pictures sketched by past research.
“Even in earlier experiments, scientists noticed that power generation in micro solid oxide fuel cells varies greatly depending on the structure of such cells. In the experiment with the strain of the ion conductor, we have now found a possible explanation for this behavior,” senior author Jennifer Rupp says in the release, which continues: “It now appears possible to optimize the characteristics of ion-conducting membranes. This supports the development of future gas sensors, ion-based data storage and micro energy converters, such as fuel cells—and potentially a range of other as yet unknown applications in the promising field of ionics.”
In addition to ion conductivity, wrinkles can also help improve and widen industrial applications of thin film membranes for filtration, according to other new research from Imperial College London. Imperial researchers engineered thin polyamide nanofilms with crumples that increase the filter’s surface area. The thinness of the nanofilms—less than 10 nm—also helps speed membrane filtration, according to the Science paper describing the results.
“Membranes are currently used for a range of important tasks such as making water drinkable and life-saving kidney filtering,” coauthor Andrew Livingston says in an Imperial College press release. “The drawback has been that industry hasn’t been able to use membranes in organic liquid systems more widely because they’ve had cost and design limitations. Our research suggests that we can overcome these challenges, which could make these membranes useful for industries ranging from pharmaceutical companies to oil refining. The energy and environmental benefits could be massive.”
To make stable composite membranes, the team layered the wrinkly nanofilms on an alumina support. The ceramic-support membrane is surprisingly strong and stable in a range of organic solvents, which is notable because lack of stability has limited previous industrial membranes. The membrane can filter organic liquids at a pressure of 50 bar and can do so 400 times faster than conventional membranes, according to the release.
Moreover, the film was able to effectively filter a solution of solvent, alcohol, and dyed molecules at high pressures, only letting the alcohol pass through a simple dead-end filter.
The press release extrapolates the impact of the research. “Ultimately, the researchers believe that their prototype membrane could be used to improve or completely replace industrial processes that process organic solvents, which currently rely on evaporation and distillation techniques. Approximately 30 percent of the world’s energy is currently used by industry, with a substantial fraction of that being used in evaporation and distillation processes. These industries could potentially make major energy savings if they used the membranes, with consequent reductions in carbon dioxide emissions.”
The ion conductivity paper, published in Nature Materials, is “The effect of mechanical twisting on oxygen ionic transport in solid-state energy conversion membranes” (DOI: 10.1038/nmat4278).
The filtration paper, published in Science, is “Sub–10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation” (DOI: 10.1126/science.aaa5058).